- Title
-
Treponema pallidum promoted microglia apoptosis and prevented itself from clearing by human microglia via blocking autophagic flux
- Authors
- Hu, Y.T., Wu, K.X., Wang, X.T., Zhao, Y.Y., Jiang, X.Y., Liu, D., Tong, M.L., Liu, L.L.
- Source
- Full text @ PLoS Pathog.
|
The number of microglia in |
|
The percentage of apoptotic cells was significantly increased in HMC3 cells treated with |
|
RNA sequencing revealed that The gene transcription of HMC3 cells treated with |
|
A tandem fluorescent-labelled plasmid pmCherry-EGFP-LC3B construct was transfected into HMC3 cells to quantify the numbers of different LC3 puncta and to assess the impact of |
|
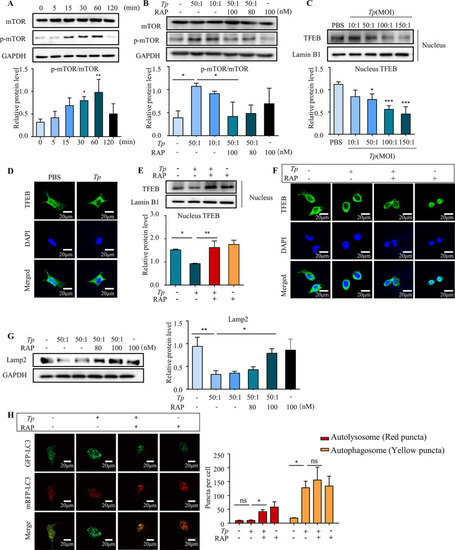
To assess mTOR activation in |
|
The accumulation of autophagosomes is associated with the mechanism by which (A) HMC3 cells were treated with |
|
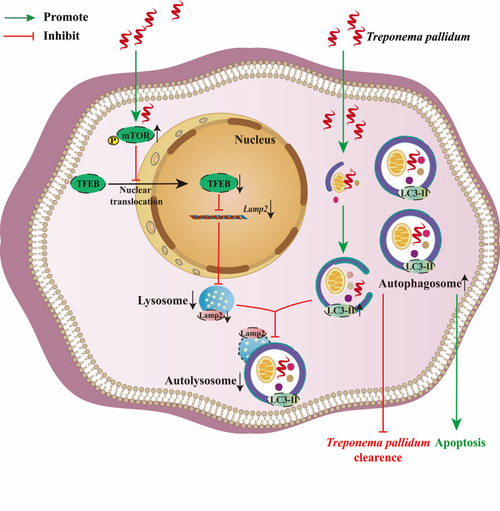
In this study, we demonstrated that |