- Title
-
3D cell segregation geometry and dynamics are governed by tissue surface tension regulation
- Authors
- Méhes, E., Mones, E., Varga, M., Zsigmond, Á., Biri-Kovács, B., Nyitray, L., Barone, V., Krens, G., Heisenberg, C.P., Vicsek, T.
- Source
- Full text @ Commun Biol
|
Characterization of homotypic contact strength by dual pipette aspiration assay. |
|
3D segregation of model cell types. Representative epifluorescent images from image series of segregation experiments. |
|
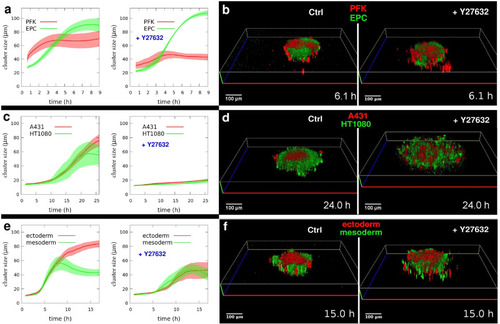
3D segregation dynamics depends on actomyosin contractility. Quantitative analysis of segregating homotypic cell domain sizes. |
|
Aggregation dynamics depend on non-muscle myosin 2 assembly. Quantitative analysis of aggregation of A431 cells. |
|
Spatial positioning during segregation depends on non-muscle myosin 2 assembly and function. |
|
Schematic summary of experimentally studied aspects of tissue surface tension regulation. |
|
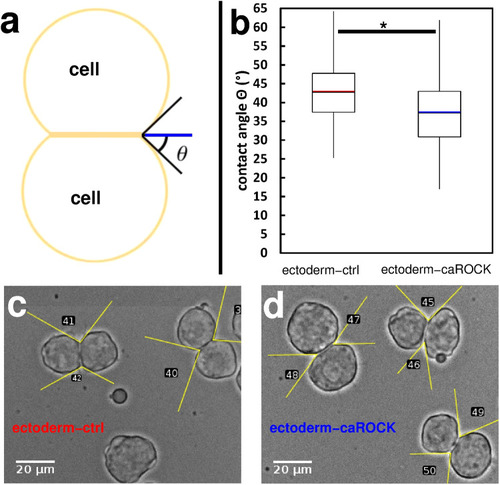
Contact formation is influenced by the regulation of actomyosin contractility. |
|
Aggregation dynamics is influenced by regulation of actomyosin contractility. Quantitative analysis of aggregation of zebrafish ectoderm cells. |
|
Spatial configuration of segregated domains depends on actomyosin contractility regulation. |