- Title
-
β-Catenin Elicits Drp1-Mediated Mitochondrial Fission Activating the Pro-Apoptotic Caspase-1/IL-1β Signalosome in Aeromonas hydrophila-Infected Zebrafish Macrophages
- Authors
- Sharma, S., Kumar, M., Kumar, J., Mazumder, S.
- Source
- Full text @ Cells
|
|
|
|
|
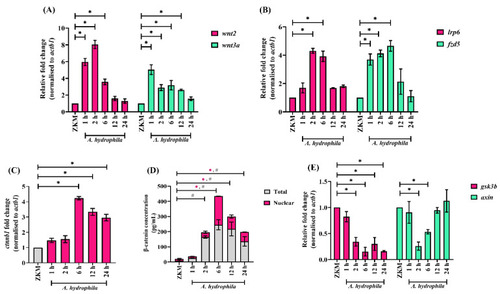
Canonical Wnt signaling is activated in |
|
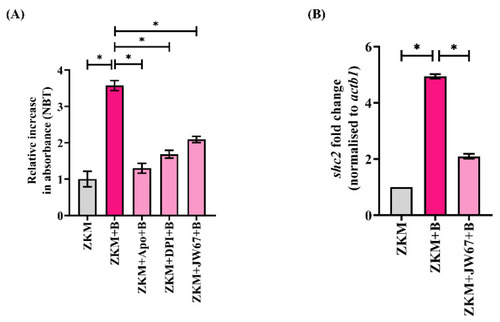
β-catenin triggers NOX-induced ROS production and upregulates |
|
β-catenin triggers ROS-induced-mtROS generation and downregulates |
|
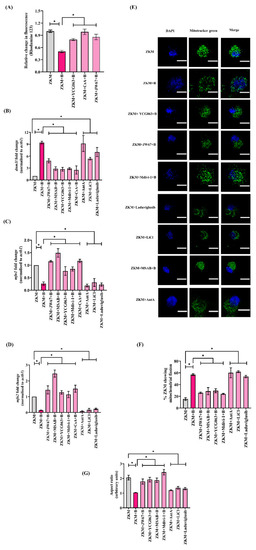
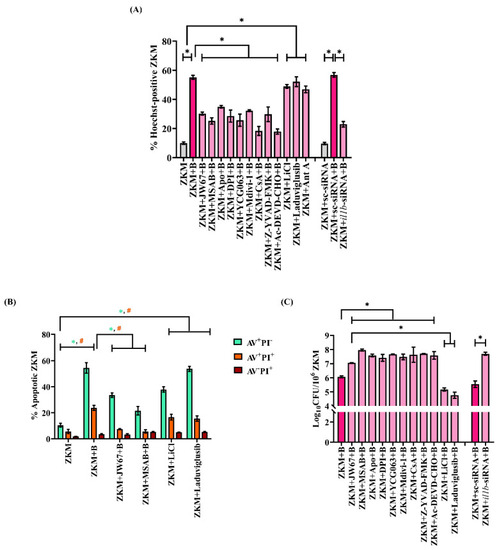
β-catenin-induced mtROS prompts ΔΨm loss leading to Drp1-mediated mitochondrial fission in |
|
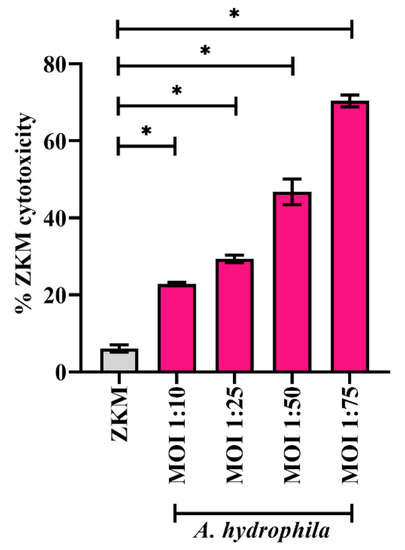
β-catenin plays a pro-apoptotic and anti-bacterial role in |
|
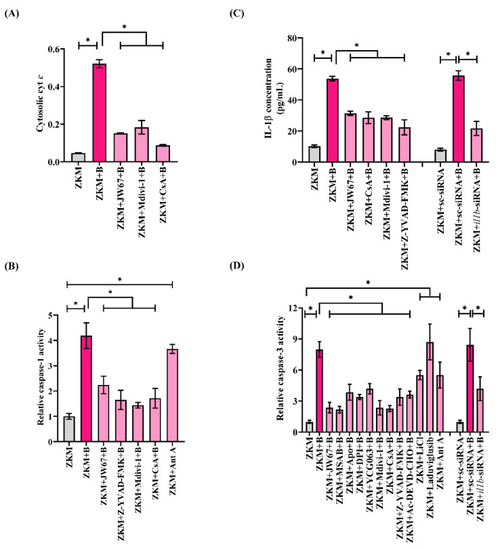
β-catenin-induced mitochondrial fission triggers cyt |
|
Overview of the study. Canonical Wnt signaling-induced NOX-mediated ROS triggers mtROS generation leading to downstream ΔΨm loss. ΔΨm loss prompts the activation of Drp1-mediated mitochondrial fission. Activated mitochondrial fission leads to cyt |