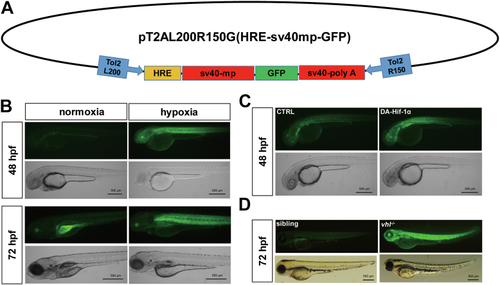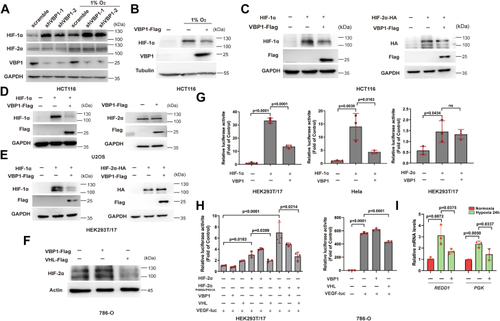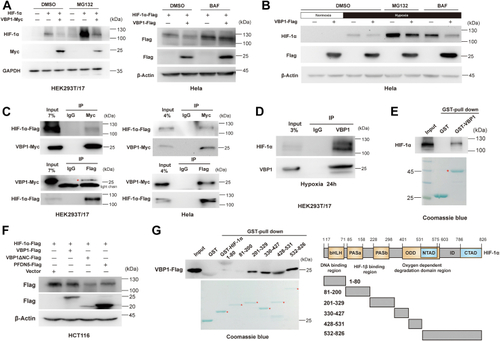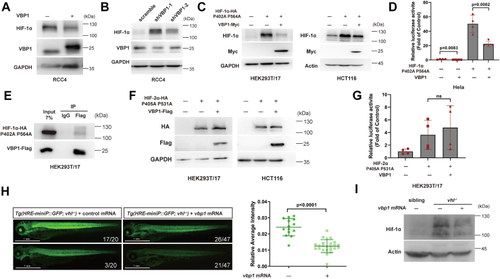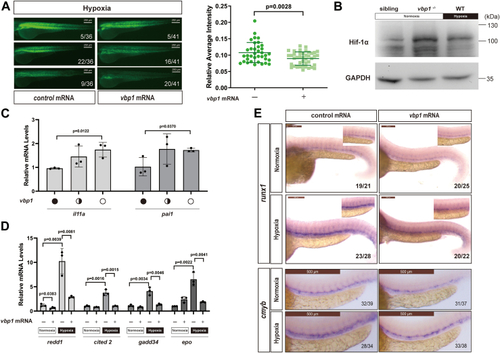
Vbp1 negatively regulates the HIF-1α signaling pathway in zebrafish.A, Representative images show GFP fluorescence in the Tg (hre-sv40mp:GFP) embryos that were injected with mCherry or vbp1 mRNA at the 1-cell stage and exposed to hypoxia for 48 h from 24 hpf. The histograms show average GFP fluorescence intensity in the somite of the Tg(hre-sv40mp:GFP) larvae (n ≥ 36) at 72 hpf. B, Western blot shows Hif-1α protein levels in the sibling, vbp1−/−, wt (hypoxia treatment) larvae at four dpf. As shown, Hif-1α is upregulated in the vbp1 mutants. C, The relative mRNA levels of hypoxia-responsive genes, namely, il11a, and pai1 in the WT, vbp1+/−, and vbp1−/− larvae. The data show upregulation of these hypoxia-responsive genes in the vbp1 mutant zebrafish (n = 3, biologically independent extracts). D, Effect of forced expression of vbp1 on redd1, cited2, gadd34, and epo mRNA expression. Embryos injected with GFP or vbp1 capped mRNA was raised to 24 hpf and then exposed to hypoxia (10% O2) for 24 h. The mRNA levels of the indicated genes were determined by qPCR. (n = 3, biologically independent extracts). E, Representative images show both runx1 and cmyb expression in the zebrafish embryos injected with control or vbp1 mRNAs under normoxic and hypoxic conditions. As shown, runx1/cmyb expression is reduced in the vbp1-injected embryos under hypoxia conditions. The brightfield images of WISH for runx1/cmyb expression are shown in the WT embryos at 36 hpf under normoxia and hypoxia exposure for 8 h starting at 28 hpf (lateral views). Scale bar, 250 μm and 500 μm representatively. All data are represented as means ± SD. n/n, number of embryos showing representative phenotype/total number of embryos examined. HIF-1, Hypoxia-inducible factor-1; pVHL, von Hippel-Lindau protein; VBP1, pVHL binding protein 1.
|