- Title
-
Evaluation of phages and liposomes as combination therapy to counteract Pseudomonas aeruginosa infection in wild-type and CFTR-null models
- Authors
- Cafora, M., Poerio, N., Forti, F., Loberto, N., Pin, D., Bassi, R., Aureli, M., Briani, F., Pistocchi, A., Fraziano, M.
- Source
- Full text @ Front Microbiol
|
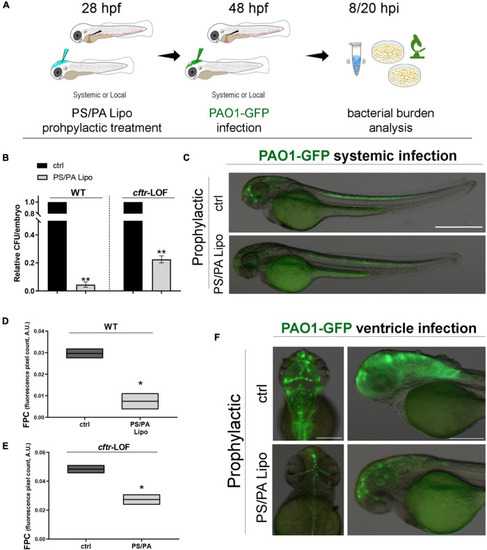
Antimicrobial activity of phosphatidylserine/phosphatidic acid (PS/PA) liposome prophylactic administration in wild-type (WT) and |
|
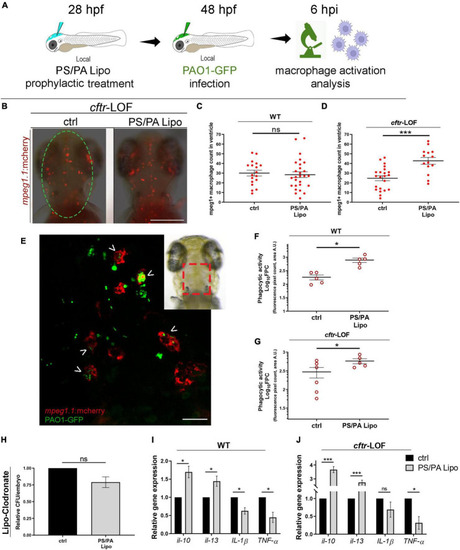
Macrophage activation in wild-type (WT) and |
|
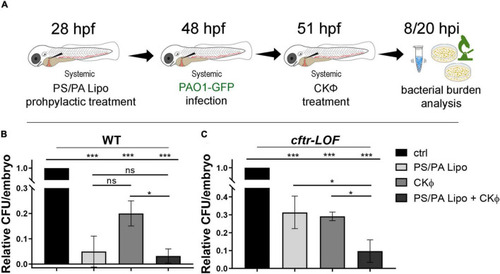
Antimicrobial activity in WT and |
|
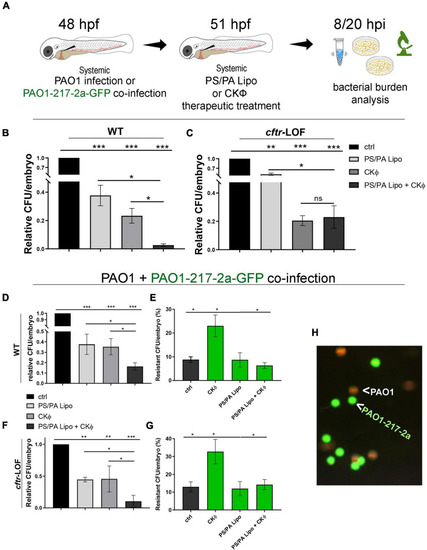
Combination treatment with phosphatidylserine/phosphatidic acid (PS/PA) liposome and CKΦ in therapeutic administration elicits synergistic effect in antimicrobial activity in wild-type (WT) and decreases phage-resistant PAO1 proliferation in |
|
PS/PA liposomes and CKΦ administration does not elicit toxic effects on human CF cells. |