- Title
-
Cratoxylumxanthone C, a natural xanthone, inhibits lung cancer proliferation and metastasis by regulating STAT3 and FAK signal pathways
- Authors
- Li, Y., Wang, H., Liu, W., Hou, J., Xu, J., Guo, Y., Hu, P.
- Source
- Full text @ Front Pharmacol
|
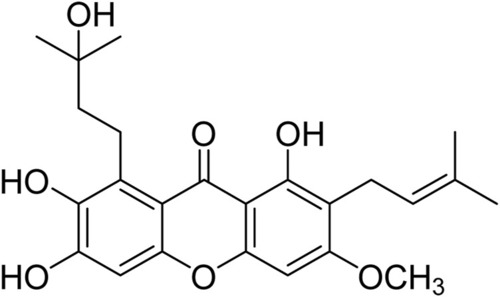
Chemical structure of cratoxylumxanthone C. |
|
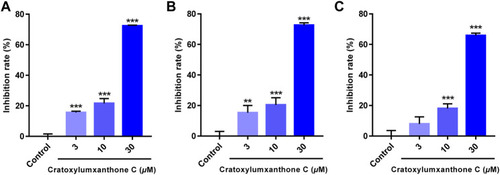
Cratoxylumxanthone C inhibited the proliferation and viability of three human cancer cell lines. A549 |
|
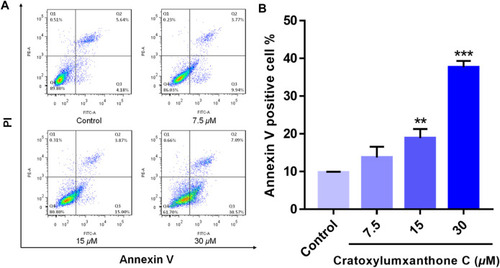
Cratoxylumxanthone C induced apoptosis in A549 cells. A549 cells were treated with different concentrations (7.5, 15, and 30 |
|
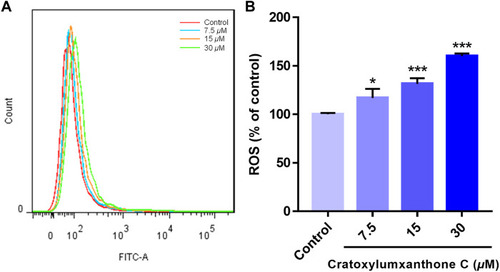
Cratoxylumxanthone C increased ROS production in A549 cells. A549 cells were treated with different concentrations (7.5, 15, and 30 |
|
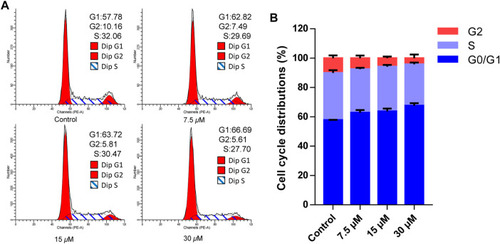
Cratoxylumxanthone C arrested G0/G1 phase in A549 cells. A549 cells were treated with different concentrations (7.5, 15, and 30 |
|
Cratoxylumxanthone C regulated STAT3 signaling pathway and apoptosis-related proteins. |
|
Cratoxylumxanthone C inhibited migration in A549 cells and regulated FAK/MMP2 signaling pathway. |
|
Cratoxylumxanthone C inhibited proliferation and migration of A549 cells in zebrafish xenografts. CM-DiI stained A549 cells were microinjected into 2 dpf zebrafish embryos. After 4 h, tumor-bearing embryos were treated with cratoxylumxanthone C (2.5, 5, and 10 |
|
The embryos from transgenic zebrafish PHENOTYPE:
|