- Title
-
Acetaminophen Disrupts the Development of Pharyngeal Arch-Derived Cartilage and Muscle in Zebrafish
- Authors
- Glasco, D.M., Wang, Z., Kang, S., Funkhouser, A.T.
- Source
- Full text @ J Dev Biol

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
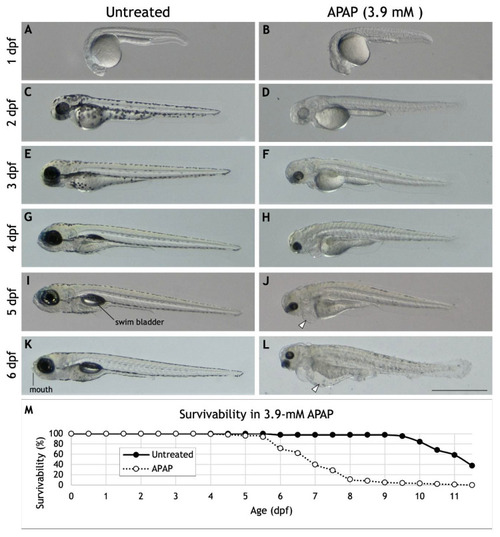
Morphology and survivability of APAP-treated larvae. ( |
|
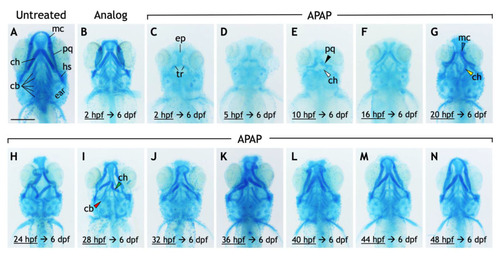
Exposure to acetaminophen, but not a non-hepatoxic analog, disrupts the development and morphology of craniofacial cartilages. ( PHENOTYPE:
|
|
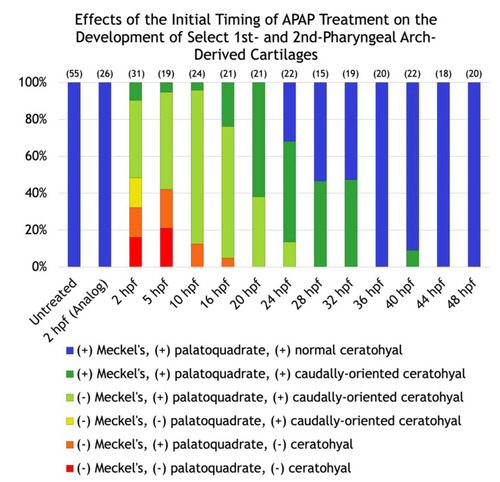
Effects of the initial timing of APAP treatment on the development of select first and second pharyngeal arch-derived cartilages. A quantification of the defects shown in |
|
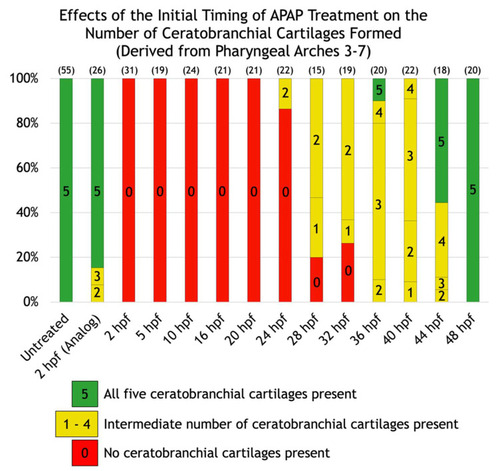
Effects of the initial timing of APAP treatment on the number of ceratobranchial cartilages formed (derived from pharyngeal arches three through seven). A quantification of the defects shown in |
|
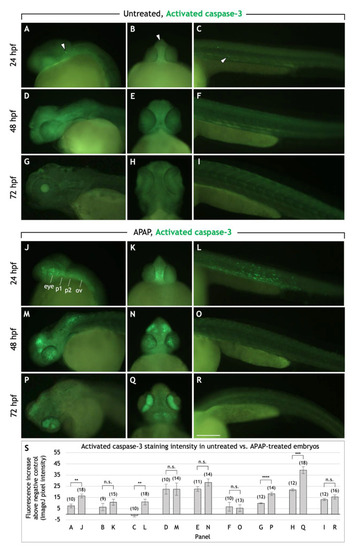
APAP treatment increases apoptosis in the head and pharyngeal arches before and during cartilage differentiation. Lateral and rostroventral views of the head and trunk regions in 24, 48, and 72 hpf embryos processed for anti-activated caspase-3 immunohistochemistry to detect apoptotic cells. In untreated embryos, apoptotic cells are sparsely distributed in the head and trunk (white arrowheads in ( PHENOTYPE:
|
|
Neural crest cell markers sox9a and sox10 are expressed in trunk and cranial neural crest cells in APAP-treated embryos. ( EXPRESSION / LABELING:
PHENOTYPE:
|
|
Most APAP-treated embryos express collagen type II, a marker for differentiated cartilage, in the craniofacial region. ( EXPRESSION / LABELING:
PHENOTYPE:
|
|
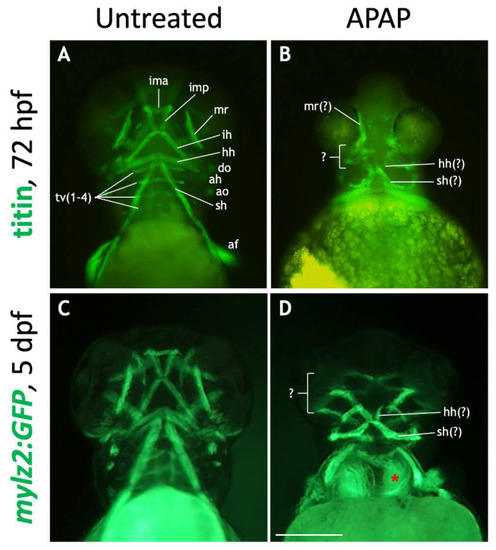
Many craniofacial muscles are absent or disorganized in APAP-treated larvae. ( EXPRESSION / LABELING:
PHENOTYPE:
|
|
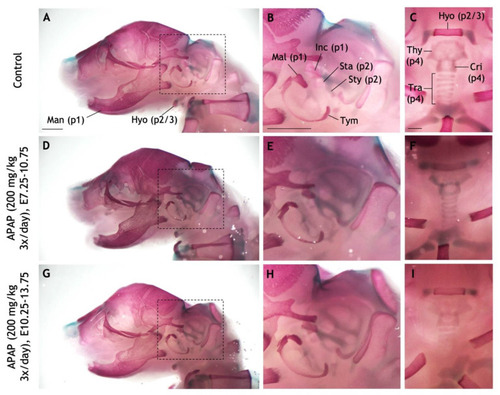
Repeated administration of high APAP dosage to pregnant mice does not recapitulate craniofacial defects. ( |