|
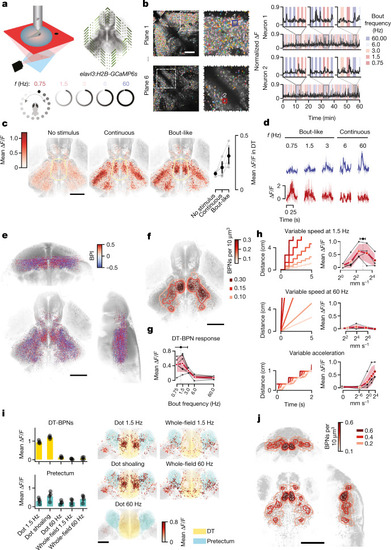
Fish-like motion activates a conserved social behaviour network.a, Schematic of stimulus presentation for activity mapping. b, Attraction towards stimuli shown in a. n = 17 (no stimulus) or n = 9 (continuous; bout-like) single animals; and n = 8 animals tested in 4 pairs (conspecific). Data are mean (black dots) ± 1 s.d. Exact P values were calculated using two-tailed t-tests compared with the no-stimulus group: P = 0.16 (continuous); P = 5.2 × 10−8 (bout-like); P = 8.1 × 10−11 (conspecific). Bonferroni-corrected α values: NS, P > 0.05/3 (NS); ***P < 0.001/3. c, Representative slices of maximum-intensity-normalized c-fos signal merged across all 28 registered animals. The views are horizontal (top row), sagittal (bottom left) and coronal (bottom right). The solid grey lines indicate the corresponding planes across the slices. The dashed line indicates the midline. Coloured patches indicate activity clusters (Extended Data Fig. 1). A, anterior; D, dorsal; L, lateral. d, The average normalized c-fos signal at the three representative horizontal planes indicated in c. n = 6 (no stimulus), n = 8 (continuous), n = 6 (bout-like) and n = 8 (conspecific) animals. e, The effect size (Cohen’s d) of normalized bulk c-fos induction compared with the no-stimulus condition. Negative values indicate a lower signal compared with the no-stimulus condition. The dendrogram represents hierarchical clustering. Statistical analysis was performed using two-tailed t-tests in each activity cluster versus the no-stimulus group. *P < 0.05/3, **P < 0.01/3, ***P < 0.001/3 (α values were Bonferroni-corrected per activity cluster). Animal numbers are the same as in d. Additional statistical information is provided as Source Data. Scale bars, 200 µm. DT, dorsal thalamus; En, entopeduncular nucleus; Hc1, caudal hypothalamus 1; Hc2, caudal hypothalamus 2; Hc3, caudal hypothalamus 3; Hi1, intermediate hypothalamus 1; Hi2, intermediate hypothalamus 2; Hi3, intermediate hypothalamus 3; Hrl, rostral hypothalamus, lateral; mHr, rostral hypothalamus, medial; MOd, medulla oblongata, dorsal; MOi, medulla oblongata, intermediate; nMLF, nucleus of the medial longitudinal fasciculus; OB, olfactory bulb; P, pallium; Pl, pallium, lateral; PM, magnocellular preoptic nucleus; Pn, pineal; PPa, anterior parvocellular preoptic nucleus; PPp, posterior parvocellular preoptic nucleus; Pr, pretectum; PT, posterior tuberculum; Ri, inferior raphe; SPd, subpallium, dorsal; SPv, subpallium, ventral; TeOa, tectum, anterior; TeOd, tectum, dorsal; TeOv, tectum, ventral; Tg, lateral tegmentum; TS, torus semicircularis; VT, ventral thalamus. Source data
|