- Title
-
The distal C terminus of the dihydropyridine receptor β1a subunit is essential for tetrad formation in skeletal muscle
- Authors
- Dayal, A., Perni, S., Franzini-Armstrong, C., Beam, K.G., Grabner, M.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
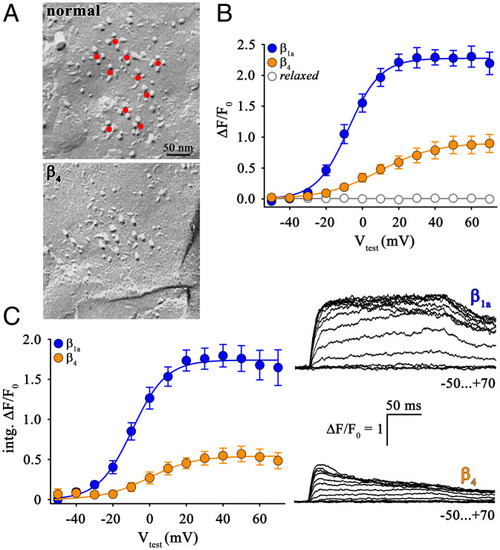
Absence of DHPR tetrad restoration in β4-expressing |
|
Loss-of-function β1a/β4 chimeras revealed the importance of the β1a C terminus in skeletal muscle DHPR–RyR1 coupling. ( |
|
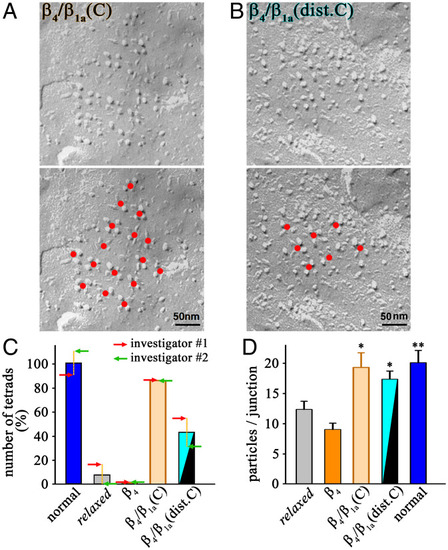
Length of the β4 C terminus is not crucial for skeletal muscle DHPR–RyR1coupling. ( |
|
The distal C terminus of β1a is crucial for skeletal muscle EC coupling. ( |
|
The distal C terminus of β1a is crucial for DHPR tetrad formation. ( |
|
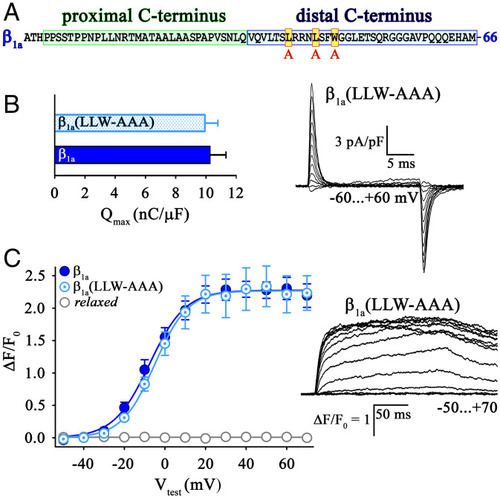
Hydrophobic residues (L496L500W503) in the β1a distal C terminus are not important for skeletal muscle EC coupling. ( |
|
Model of conformational modification of α1S by the β1a distal C terminus—prerequisite for proper skeletal muscle EC coupling. ( |