- Title
-
High calorie diet results in reversible obesity-related glomerulopathy in adult zebrafish regardless of dietary fat
- Authors
- Zeitler, E.M., Jennette, J.C., Flythe, J.E., Falk, R.J., Poulton, J.S.
- Source
- Full text @ Am. J. Physiol. Renal Physiol.
|
Figure 1.High calorie feeding caused higher body mass without fasting hyperglycemia. A: zebrafish fed high-calorie diets (high-calorie diet and high-calorie, high-fat diet) were heavier than those fed a normal diet after 8 wk. B: standard length increased over the course of the experiment in all groups except for the normal diet-fed group and was significantly longer in the high-calorie diet-fed group (vs. the normal diet-fed group) at 8 wk. C: body mass index (BMI) was significantly higher in the high-calorie, high-fat diet-fed group than in the normal diet-fed group at 8 wk. D: fasting glucose was not significantly different between the diet-fed groups. E: kidney area as measured following fixation and dissection. High-calorie diet-fed fish had significantly larger kidneys, and high-fat diet-fed fish had significantly smaller kidneys. There was no difference in relative kidney size when kidney size was normalized to total fish weight. F: lateral views of zebrafish in each group revealed increased abdominal girth compared with head size in the high-calorie diet-fed and high-calorie, high-fat diet-fed groups. G: ventral view of the kidney at the time of dissection. The kidney is outlined in black, adherent to the dorsal abdominal wall with all other abdominal organs removed. Representative images are shown, and all images were taken from the same distance with equal magnification. Scale bars = 1 cm. n = 11–15 fish/group for A–G. Data are presented as means ± SD. Statistical tests were as follows: Welch ANOVA with Dunnett’s multiple comparisons test. *P < 0.05 and **P < 0.01. HC, high-calorie diet; HCHF, high-calorie, high-fat diet; HF, high-fat diet; ND, normal diet. PHENOTYPE:
|
|
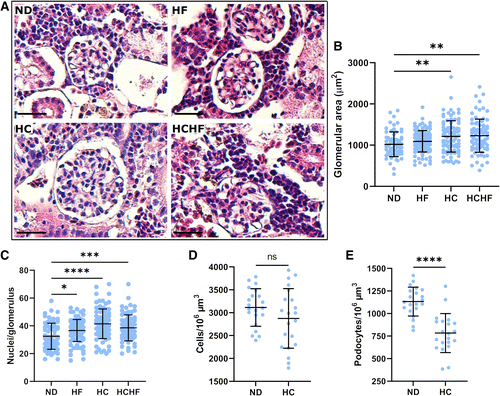
Figure 2.High-calorie diet led to glomerulomegaly and decreased podocyte density. A and B: hematoxylin and eosin-stained paraffin kidney sections revealed marked glomerulomegaly in zebrafish fed a high-calorie diet, without evidence of glomerular sclerosis. Kidneys from the high-calorie and high-calorie, high-fat diet-fed groups had significantly larger glomeruli than those in the normal diet-fed group. C: as evaluated by light microscopy, the high-calorie diet-fed group had a higher total cell number in each glomerulus compared with normal diet-fed controls. D: confocal microscopy of kidneys from transgenic fish also showed no difference in total cell density between fish fed a normal diet and fish fed a high-calorie diet. E: in contrast, glomeruli from fish fed a high-calorie diet had lower podocyte density compared with fish fed a normal diet. Scale bars = 25 µm. In B and C, at least 60 glomeruli from 3–4 fish/group were measured on three sections per fish. In D and E, n = 21 glomeruli per group. Data are presented as means ± SD. Statistical tests were as follows: Welch ANOVA with Dunnett’s multiple comparisons test. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. HC, high-calorie diet; HCHF, high-calorie, high-fat diet; HF, high-fat diet; ND, normal diet. PHENOTYPE:
|
|
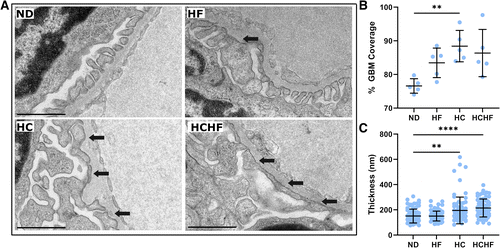
Figure 3.Electron microscopy reveals foot process effacement and glomerular basement membrane (GBM) thickening in the setting of high-calorie diets. A: transmission electron micrographs of representative glomeruli from each diet-fed group revealed foot process effacement in both groups fed higher-calorie diets. Arrows indicate areas of foot process effacement. B: the percentage of the GBM covered by intact podocyte foot processes increased in the high-calorie diet-fed group, suggesting loss of the slit diaphragm area. C: glomeruli from high-calorie and high-calorie, high-fat diet-fed fish had increased GBM thickness. Scale bars = 1 µm. In B, n = 5 electron micrographs from each group. In C, GBM thickness was measured at 15 randomly assigned points in each of five images. Data are presented as means ± SD. Statistical tests were as follows: Welch ANOVA with Dunnett’s multiple comparisons test. **P < 0.01 and ****P < 0.0001. HC, high-calorie diet; HCHF, high-calorie, high-fat diet; HF, high-fat diet; ND, normal diet. PHENOTYPE:
|
|
Figure 4.High-calorie diets caused proximal tubule enlargement and ectopic lipid accumulation. A and B: hematoxylin and eosin-stained sections from fish fed high-fat and high-calorie diets (high-fat, high-calorie, and high-calorie, high-fat diets) revealed pale cytoplasm with enlargement of the proximal tubule and proximal tubular lumen. The cross-sectional area of the proximal tubules in the high-fat and high-calorie diet-fed fish were significantly larger than those fed a control diet. C: fish fed either high-calorie diet developed ectopic lipid accumulation and brush border abnormalities. Representative electron micrographs of the apical membrane revealed a reduced and abnormal brush border (arrowheads) and copious lipid-containing vesicles (arrows). Scale bars = 25 µm in A and 1 µm in C. In B, at least 100 tubular cross-sections were measured from each group. Data are presented as means ± SD. Statistical tests were as follows: Welch ANOVA with Dunnett’s multiple comparisons test. **P < 0.01 and ****P < 0.0001. HC, high-calorie diet; HCHF, high-calorie, high-fat diet; HF, high-fat diet; ND, normal diet. PHENOTYPE:
|
|
Figure 5.High-calorie and high-calorie, high-fat diets led to ectopic lipid deposition and displacement of organelles. Transmission electron microscopy of proximal tubules from fish fed the normal-calorie diets (normal diet and high-fat diets) showed evenly spaced cells with elongated mitochondria distributed throughout the cell and few endocytic vesicles. In contrast, proximal tubule cells in the high-calorie diets (high-calorie and high-calorie, high-fat diets) had bulging apical membranes and cytoplasm filled with lipid droplets, displacing the nucleus and mitochondria to the basal portion of the cell. Scale bars = 5 µm. HC, high-calorie diet; HCHF, high-calorie, high-fat diet; HF, high-fat diet; ND, normal diet. PHENOTYPE:
|
|
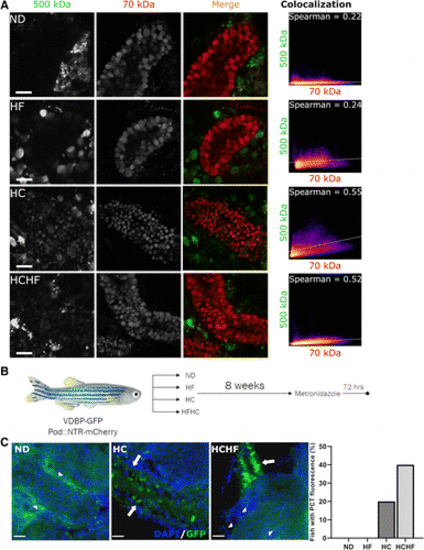
Figure 6.High-calorie diets led to glomerular filtration barrier dysfunction and susceptibility to injury. A: high-calorie diet feeding resulted in filtration barrier dysfunction. In normal diet-fed fish, no signal was present from 500-kDa dextran uptake in proximal tubules, whereas 70-kDa dextran was filtered and reabsorbed. In high-calorie and high-calorie, high-fat diet-fed fish, the signal from the larger dextran colocalized with the smaller, indicating failure of size exclusion at the glomerulus. Colocalization was demonstrated by Spearman’s ranked correlation coefficient, with 1 = absolute colocalization and 0 = no colocalization. Scatterplots demonstrate the relative intensities of red (x-axis) versus green (y-axis) pixels. B: schematic representation of dietary preconditioning to metronidazole (MTZ) injury. Zebrafish were fed one of four diets for 8 wk prior to treatment with 2 mM MTZ for 24 h and were then allowed to recover for 72 h before euthanasia. C: proximal tubule green fluorescent protein (GFP) fluorescence (arrows) was present after low-dose MTZ injury in fish preconditioned with a high-calorie diet (high-calorie or high-calorie, high-fat diets) but not in those fed a normal diet (arrowheads indicate normal tubules). The high-fat diet-fed group is not shown, as effects were equivalent to normal diet. No fish in the normal diet- or high-fat diet-fed groups had observable GFP in the proximal tubules, while 20% and 40% of those in the high-calorie and high-calorie, high-fat diet-fed groups did, respectively. In C, 5 fish/group were evaluated. Data are presented as percentages of fish with GFP in the proximal tubules. Scale bars = 20 µm. HC, high-calorie diet; HCHF, high-calorie, high-fat diet; HF, high-fat diet; ND, normal diet. PHENOTYPE:
|
|
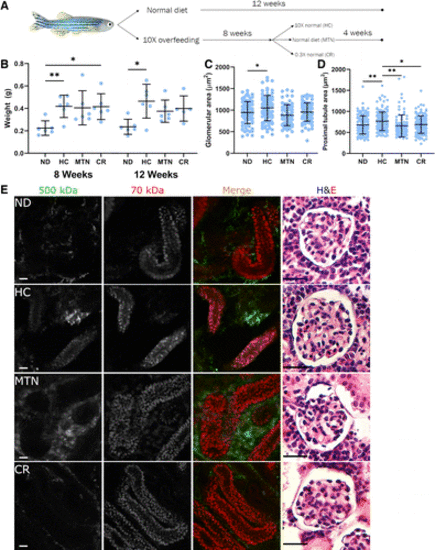
Figure 7.Calorie restriction ameliorated the pathological and functional changes caused by high-calorie diets. A: schematic of overfeeding and calorie restriction. Zebrafish were fed a high-calorie diet for 8 wk and then either continued on the high-calorie diet or transitioned to maintenance calories or calorie restriction for 4 wk and compared with fish fed a control diet throughout the 12 wk. B: high-calorie diet induced significant weight gain. Maintenance and calorie restriction diets resulted in slight weight loss, whereas continued high calorie feeding resulted in continued weight gain. C: glomerulomegaly receded with 4 wk of either maintenance or calorie restriction diet, with glomerular size similar to that of control diet-fed fish. High-calorie diet-fed fish continued to exhibit glomerulomegaly. At least 70 glomeruli from 3–4 fish/group were measured on hematoxylin and eosin (H&E)-stained sections. D: proximal tubular enlargement also returned to normal after 4 wk of maintenance or calorie restriction diet. At least 100 proximal tubules from 3−4 fish/group were measured on H&E-stained slides. E: filtration barrier function was restored after 4 wk of either maintenance or calorie restriction diet. High-calorie diet feeding resulted in the presence of 500-kDa dextran in the proximal tubules and glomerulomegaly at 12 wk. Reducing calories to maintenance or calorie-restricted levels eliminated dysfunctional filtration and resulted in normalization of glomerular size. Scale bars = 20 µm. In B, n = 6 fish/group. In C, >60 glomeruli per group were measured per group. In D, >100 tubular cross sections per group were measured. Data are presented as means ± SD. Statistical tests were as follows: Welch ANOVA with Dunnett’s multiple comparisons test. *P < 0.05 and **P < 0.01. CR, calorie-restrict diet; HC, high-calorie diet; MTN, maintenance diet; ND, normal diet. |