- Title
-
Zebrafish models of Alx-linked frontonasal dysplasia reveal a role for Alx1 and Alx3 in the anterior segment and vasculature of the developing eye
- Authors
- Yoon, B., Yeung, P., Santistevan, N., Bluhm, L., Kawasaki, K., Kueper, J., Dubielzig, R., Vanoudenhove, J., Cotney, J., Liao, E.C., Grinblat, Y.
- Source
- Full text @ Biol. Open
|
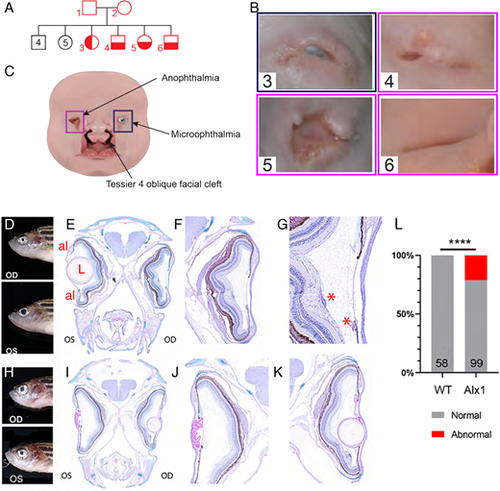
Ocular malformations are associated with ALX1/alx1 loss-of-function in humans and zebrafish. (A) Consanguineous ALX1L165F/+ parents produced 13 children, four of whom were homozygous for ALX1L165F and had complex frontonasal dysplasia (FND3) (subject numbers noted in red). Unaffected individuals did not have eye or facial phenotypes suggestive of FND. (B) Diagram summarizing the anophthalmia, coloboma and bilateral Tessier oblique facial clefts (ObFC) characteristic of FND3. (C) Human subjects displayed bilateral ObFC (not shown) and a range of ocular malformations. The eldest sibling (subject 3) presented with right coloboma and left microophthalmia. The next child (subject 4) presented with bilateral anophthalmia with fused eyelids and shallow orbits. Subject 5 presented with bilateral anophthalmia with open shallow orbits. The upper and lower eyelids were absent, exposing the orbital mucosa. The nasal alae were also malformed with nodular skin tags. Subject 6 had bilateral anophthalmia, fused eyelids, and shallow orbit, similar to subject 4. (D-L) alx1uw2016 adult zebrafish exhibit milder ocular defects, primarily in the anterior ocular segment. (D-G) A representative alx1uw2016 adult with a unilateral ocular malformation (D). (E) Transverse section through the head of the fish in E shows a normal left eye and aphakia (absent lens) on the right. (F) The right eye lacks the lens and the ventral annular ligament but is largely normal otherwise. (G) The right eye contains mononuclear inflammatory cells near the ventral iris and the optic nerve head (asterisks). (H-K) A representative alx1uw2016 zebrafish with bilateral ocular defects (H). (J) In transverse section through the head, the left eye is missing the lens (aphakia) and contains a thick, multifocally disrupted lens capsule, which is embedded in the anterior chamber. There is no annular ligament on the dorsal iridocorneal angle. (K) The right eye contains a small spherical lens nucleus inside a wrinkled lens capsule. The ventral iridocorneal angle is devoid of the annular ligament. (L) Ocular defects were observed in 21.2% of alx1uw2016 homozygotes examined, and none of the wildtype fish of a similar age (****P<0.0001, Fisher's exact test). OD: oculus dexter, right eye; OS: oculus sinister, left eye; al: annular ligament; L: lens; *, mononuclear inflammatory cells. PHENOTYPE:
|
|
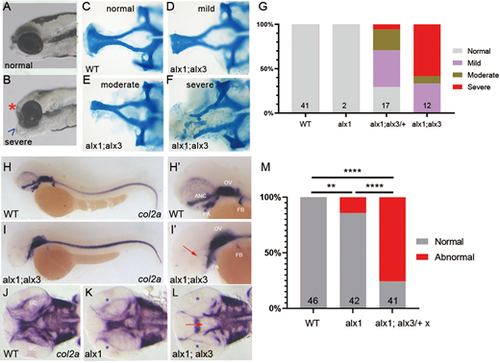
alx1 and alx3 function redundantly to control anterior neurocranium morphogenesis in zebrafish. Larvae derived from a cross between alx1;alx3/+ parents were fixed at 5 dpf. (A,B) Representative siblings with normal facial structures (A) and with aberrantly short neurocranium (asterisk) and abnormally protruding jaw cartilage (arrowhead; B). (C-F) Alcian Blue staining revealed EP anomalies that range from mild (reduced median ethmoid plate width in D), moderate (absent median and reduced lateral ethmoid plate in E), to severe (absent ethmoid plate in F). (G) Penetrance and severity of cartilage defects is increased in alx1;alx3 compared to alx1;alx3/+ embryos (two trials). (H-M) Embryos derived from wildtype, alx1, or alx1;alx3/+ parental crosses were fixed at 2 dpf and stained for col2a1a expression using whole-mount in situ hybridization. (H,H′) Normal col2a1a expression in a wildtype embryo. (I,I′) A truncated EP in an alx1;alx3 embryo (arrow). (J) Normal col2a1a expression in a wildtype embryo. (K) Normal ethmoid plate and reduced presumptive scleral staining (asterisk) in an alx1 mutant. (L) Hypoplastic ethmoid plate (arrow) and absent presumptive scleral staining (asterisk) in an alx1;alx3 mutant. ANC, anterior neurocranium; OV, optic vesicle; PA, pharyngeal arches; FB, fin bud; SC, sclera. M: 75% of the embryos derived from alx1;alx3/+ have aberrant ANC and reduced scleral precursors (**P=0.0097, ****P<0.0001, Fisher's exact test; two trials for wildtype and alx1;alx3/+, and one trial for alx1). Embryos in A,B, and H-I′ are shown in lateral views, anterior to the left. Embryos in J-L are shown in ventral views, anterior to the left. |
|
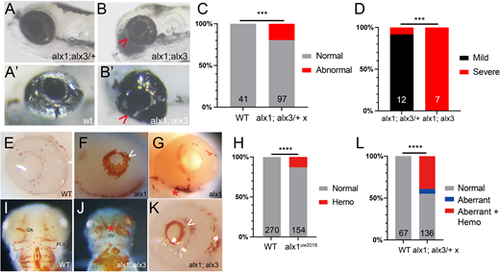
Zebrafish alx1 and alx3 function redundantly during ocular morphogenesis. (A,B) Larvae generated from a cross between alx1;alx3/+ parents were scored at 5 dpf for ocular defects. An alx1;alx3/+ larva with normal ocular morphology (A) similar to that of a wildtype embryo (A′). (B) An alx1;alx3 sibling with ocular coloboma and a misshapen eye (severe defect, arrowheads in B,B′). (C) These ocular defects are restricted to progeny of alx1;alx3/+ parents (***P=0.0009, Fisher's exact test, two trials). (D) The severe ocular defect shown in B is strongly associated with the alx1;alx3 genotype, while the majority of alx1;alx3/+ embryos present with a milder misshapen eye phenotype (not shown) (***P=0.0002 using Fisher's exact test, two trials). (E-L) Embryos derived from wildtype or alx1;alx3/+ parents were stained with o-dianisidine at 2-3 dpf to label hemoglobin in red blood cells. Normal hemoglobin distribution in the eye of a wildtype embryo (E) versus ocular hemorrhage (arrowhead in F) and periocular hemorrhage (asterisk in G) in alx1 mutants. (H) Hemorrhaging is restricted to a subset of alx1 mutants (****P<0.0001, Fisher's exact test, five trials). Normal hemoglobin distribution in the pharyngeal region of a wildtype embryo (I) versus evidence of hemorrhaging in an alx1;alx3 embryo (J). Intraocular and periocular hemorrhages (asterisk) near the optic artery and optic vein in an alx1;alx3 embryo (K). (L) Hemorrhaging is strongly associated with eye defects in alx1;alx3/+ and alx1;alx3 embryos (****P<0.0001, Chi-square test, two trials). Embryos are shown in lateral views, anterior to the left except in I and J, which are ventral views, anterior to the top. OA, optic artery; OV, optic vein; PLV, palatocerebral vein. PHENOTYPE:
|
|
alx1 and alx3 functions are dispensable for retinal ganglion cell differentiation and retinal function in zebrafish larvae. Embryos derived from wildtype or alx1;alx3/+ incrosses were fixed at 2 dpf and stained with zn5, a retinal ganglion cell (RGC) marker, and DAPI, a nuclear co-stain (A-H). (A,B) A representative wildtype eye shows strong zn5-labeled RGCs and a characteristic layered nuclear organization (of four embryos). (C,D) Normal RGC and nuclear patterns in an alx1 mutant (of two embryos). (E,F) Normal RGC and nuclear patterns in a representative alx1;alx3/+ retina (of four embryos). (G,H) Normal RGC and nuclear patterns in a representative alx1;alx3 retina (of two embryos). Left eyes are shown in lateral views, anterior to the left. Scale bar, 25 µm. (I) Diagram summarizing the dark flash assay, with a lights-off stimulus eliciting O-bend turn responses in 5 dpf larval zebrafish. (J) Mean frequency of O-bend initiations to a series of 10 dark flash stimuli was measured in larvae derived from wildtype or alx1;alx3/+ crosses, and larvae were genotyped post-hoc. n.s.: P>0.9999 versus wildtype control; ****P<0.0001 versus wildtype control. ANOVA with Kruskal–Wallis test. Error bars indicate s.e.m. |
|
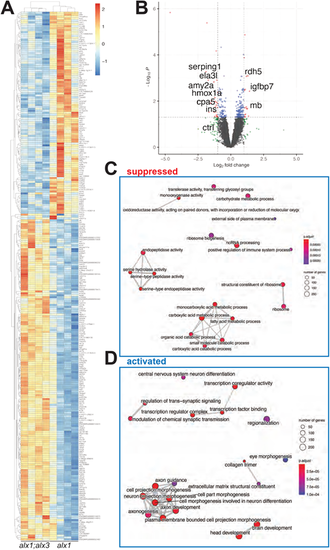
RNAseq transcriptome analysis identifies perturbation of ocular development pathways in alx1;alx3 mutants. Larvae derived from alx1uw2016;alx32113/+ parents were lysed individually at 5 dpf for RNA extraction and Illumina high-throughput sequencing. A heat map (A) of significantly dysregulated genes (FDR<0.5) in alx1uw2016;alx3uw2113 double mutants relative to alxuw2016 siblings. A volcano plot (B) of statistical significance against fold-change in alx1uw2016;alx3uw2113 mutants relative to alxuw2016 siblings. Vertical lines indicate −1-fold and +1-fold cut-offs and the horizontal line indicates the FDR=0.05 cut-off. (C,D) The top 20 enriched and top 20 suppressed terms in alx1uw2016;alx3uw2113 double mutants relative to alxuw2016 siblings, identified by GSEA. |
|
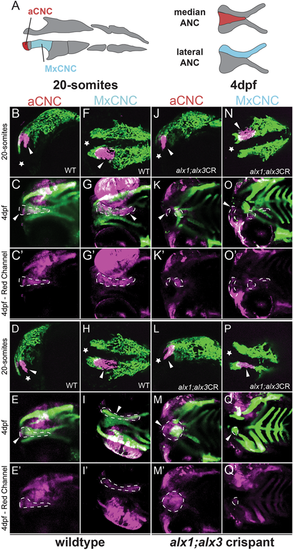
Zebrafish alx1 and alx3 functions are required in migratory anterior cranial neural crest. (A) A schematic fate map of 20-somite-stage cranial neural crest at 4 dpf. The anterior cranial neural crest (aCNC) contributes to the median element of the anterior neurocranium (ANC) while the maxillary stream of migrating CNC (MxCNC) contributes to the maxillary prominence and subsequently coalesce to form the lateral element of the ANC. B-Q: aCNC and MxCNC were labeled by kaede photoconversion (magenta) at 20 somites and imaged immediately, allowed to develop until 4 dpf and imaged again (20-somites images: star sign marks the anterior; 4 dpf images: anterior to the left). alx1-/-;alx3 crispants exhibit a midline cleft (K,M,O,Q) with malformed median and lateral elements. The aCNC and MxCNC migrating streams failed to reach their destinations and ectopically localized along the migratory path (arrowheads in K,M,O,Q). Note that ocular fluorescence is due to the presence of iridophores and retinal pigment epithelium in the eye, not to kaede fluorescence at 4 dpf. Arrowheads point to photoconverted cells and their progeny. Dotted line outlines the shape of the photoconverted cells at 4 dpf. Scale bar: 100 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
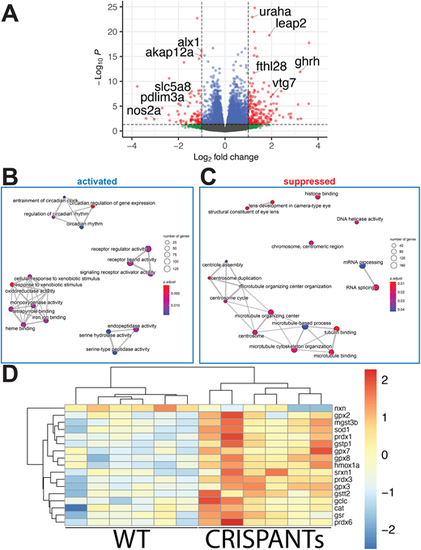
RNAseq transcriptome analysis identifies a role for alx1 and alx3 in oxidative stress response regulation.alx1uw2016;Tg(sox10:kaede) embryos were injected with alx3 CRISPR RNPs to induce somatic mutations at the alx3 locus to generate alx1;alx3CR embryos. At 3 dpf,alx1;alx3CR embryos with characteristic craniofacial defects and age-matched Tg(sox10:kaede) embryos were lysed. A total of six replicate groups of 20 embryos from each genotype were collected in two independent experiments for RNA extraction and Illumina high-throughput sequencing. (A) Volcano plot shows the distribution of genes that are differentially expressed in alx1;alx3CR;sox10:GFP embryos relative to sox10:GFP embryos at 3 dpf. Vertical lines indicate −1.5-fold and +1.5-fold change cut-offs, and the horizontal line indicates the FDR=0.05 cutoff. (B,C) molecular pathways that are significantly suppressed (B) or activated (C) in alx1;alx3CR;sox10:GFP embryos were identified using GSEA, with top 20 activated and top 20 suppressed terms shown as a 2D map to emphasize overlaps. (D) Heatmap summary of differentially expressed oxidative stress response genes shows increased expression levels of these genes in alx1;alx3CR;sox10:GFP embryos. |
|
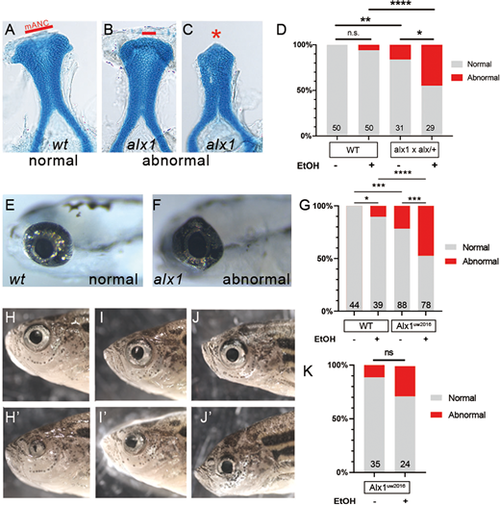
Zebrafish alx1 protects craniofacial and ocular lineages against ethanol toxicity. Embryos derived from wildtype, alx1uw2016, or alx1uw2016/+ parents were exposed to 0.5% ethanol from 6 hpf until 48 hpf and examined for craniofacial and ocular abnormalities at 5 dpf. (A-C) Embryos were stained with Alcian Blue to visualize anterior neurocranium (ANC) chondrocytes. Examples of normal ANC morphology (A) in an ethanol-treated wildtype larva, mildly dysmorphic median ANC in an ethanol-treated alx1uw2016 mutant (B), and severely abrogated ANC with missing median element in an ethanol-treated alx1uw2016 mutant. (D) Ethanol exposure does not increase penetrance of craniofacial defects in wildtype (n.s., not significant; P=0.2424) whereas it increases in progeny from alx1×alx1/+ (*P=0.0237). Compared to untreated or treated wildtype, craniofacial defects are more penetrant in untreated or progeny from alx1×alx1/+ (**P=0.0066, ****, P<0.0001), respectively. Fisher's exact test was used to measure statistical significance (three trials for wildtype and two trials for alx1×alx1/+). (E-G) Embryos were exposed to ethanol and examined for ocular anomalies at 5 dpf. (E) A representative 5 dpf wildtype larva with normal ocular morphology. (F) Misshapen eye in a representative alx1 homozygote. (G) Ethanol exposure increases penetrance of ocular abnormalities in wildtype (*P=0.0448) and in alx1uw2016 mutants (***P=0.0005). Compared to wildtype, abnormal ocular phenotype is more penetrant in both untreated alx1 mutants (***P=0.0003) and in ethanol-treated ones (****P<0.0001). Fisher's exact test was used to measure statistical significance (one trial for wildtype and two trials for alx1 mutants). (H-J) Three representative alx1uw2016 adults exposed to 0.5% ethanol from 6 hpf until 48 hpf and raised to adulthood (2 months of age) exhibit unilateral ocular anomalies. (K) Penetrance of ocular defects increased following embryonic exposure to ethanol but did not rise to significance (n.s., P=0.1022. Fisher's exact test, one trial). (A-C) dissected ANC preparations, anterior to the top. (E,F) lateral views, anterior to the left. H and H′ are different sides of the same fish, as are I and I′, and J and J′, shown in lateral view. mANC: median ANC. |