- Title
-
Angiogenesis, Anti-Tumor, and Anti-Metastatic Activity of Novel α-Substituted Hetero-Aromatic Chalcone Hybrids as Inhibitors of Microtubule Polymerization
- Authors
- Sun, M., Wang, Y., Yuan, M., Zhao, Q., Zhang, Y., Yao, Y., Duan, Y.
- Source
- Full text @ Front Chem
|
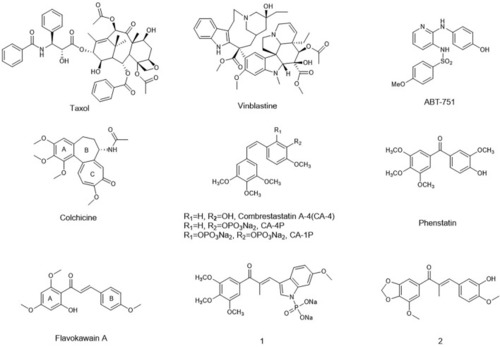
Representative tubulin inhibitors with anti- angiogenesis potency. |
|
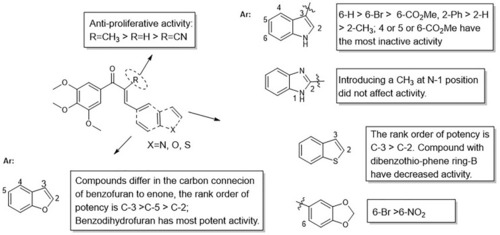
Summarized SARs of synthesized chalcone analogs. |
|
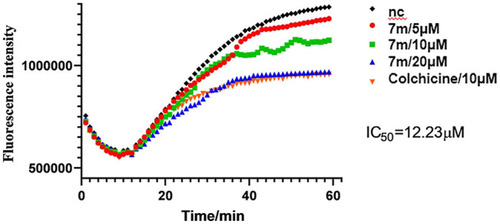
Tubulin polymerization inhibitory activity of 7m |
|
|
|
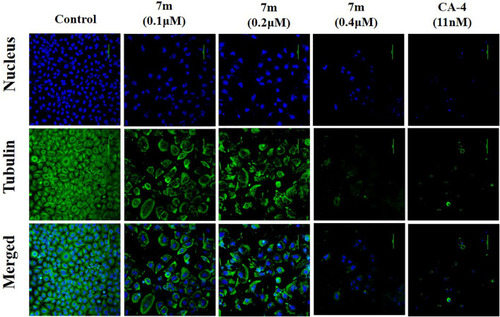
Immunofluorescence assay analysis of the effect of compound 7m on the cellular microtubule network after treatment with DMSO (control) or 7m at the indicated concentrations (0.1, 0.2, and 0.4 µM) for 24 h. Microtubules and unassembled tubulin are shown in green, and DNA is shown in blue. |
|
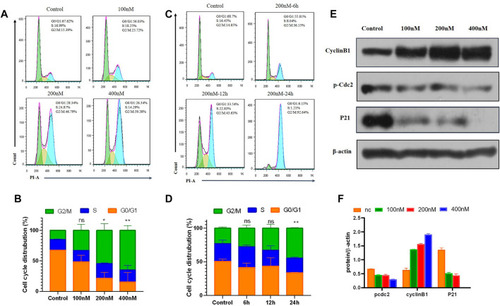
Compound 7m induced cell cycle arrest in G2/M arrest on MCF-7 cells. |
|
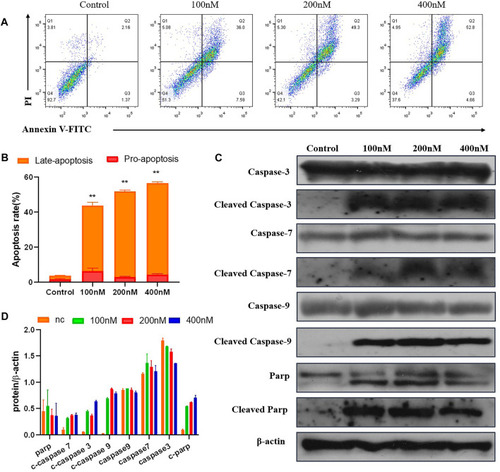
The compound 7m induces MCF-7 cell apoptosis. |
|
Effect of 7m on the human umbilical vein endothelial cells (HUVECs) migration, invasion, and tube formation. |
|
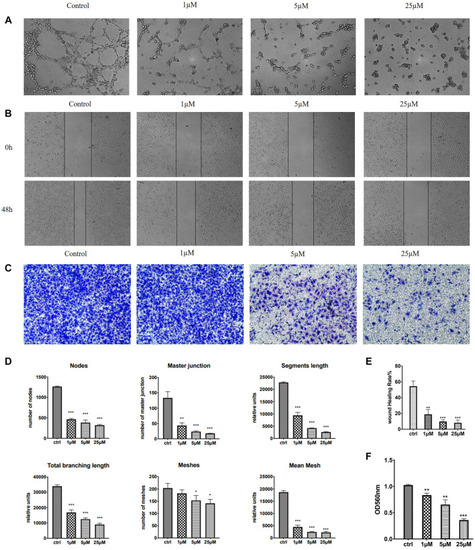
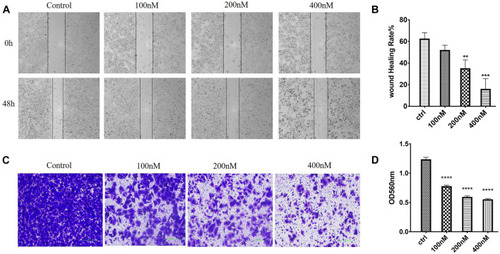
Effects of 7m on human breast adenocarcinoma (MCF-7) cell migration and invasion |
|
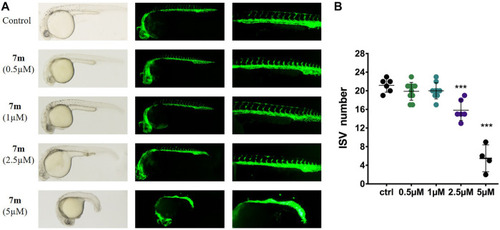
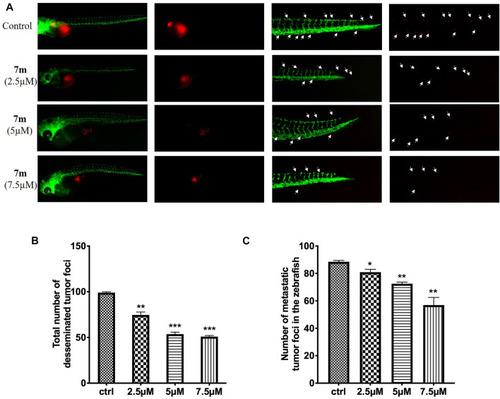
Anti-angiogenic effects of 7m in zebrafish embryos. |
|
|
|
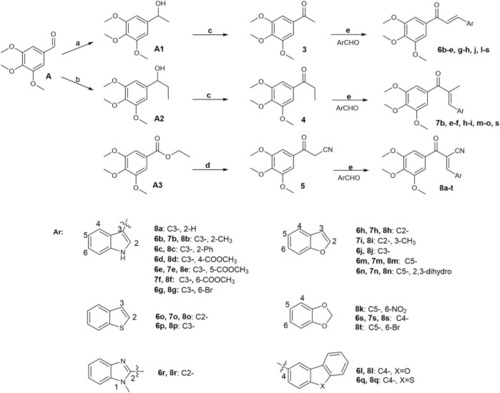
Reagents and conditions: |