- Title
-
Purkinje cells located in the adult zebrafish valvula cerebelli exhibit variable functional responses
- Authors
- Chang, W., Pedroni, A., Köster, R.W., Giacomello, S., Ampatzis, K.
- Source
- Full text @ Sci. Rep.
|
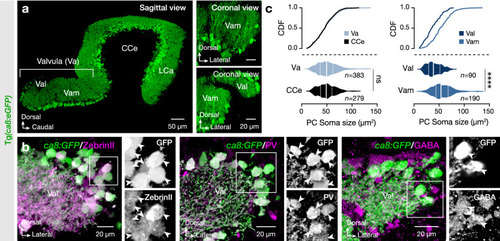
Expression pattern and specificity of |
|
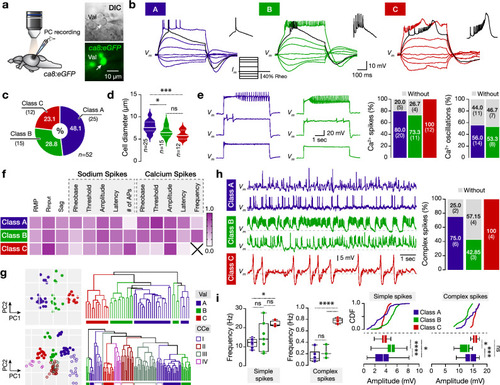
Variable firing, cellular, and spontaneous activity properties of the adult zebrafish valvular Purkinje cells. ( |
|
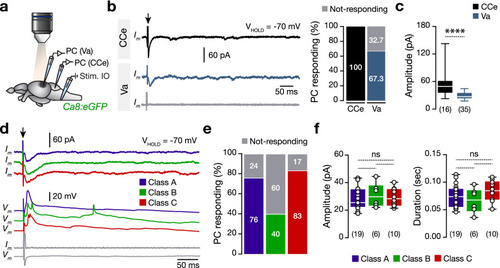
Direct electrical stimulation of inferior olive generates large-amplitude events in a proportion of valvular Purkinje cells. ( |
|
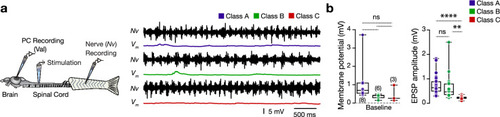
Adult zebrafish valvular Purkinje cell do not discharge during fictive locomotion. ( |