- Title
-
Knockdown of miR-26a in Zebrafish Leads to Impairment of the Anti-Inflammatory Function of TnP in the Control of Neutrophilia
- Authors
- Pimentel Falcao, M.A., Banderó Walker, C.I., Rodrigo Disner, G., Batista-Filho, J., Silva Soares, A.B., Balan-Lima, L., Lima, C., Lopes-Ferreira, M.
- Source
- Full text @ Fish Shellfish Immunol.
|
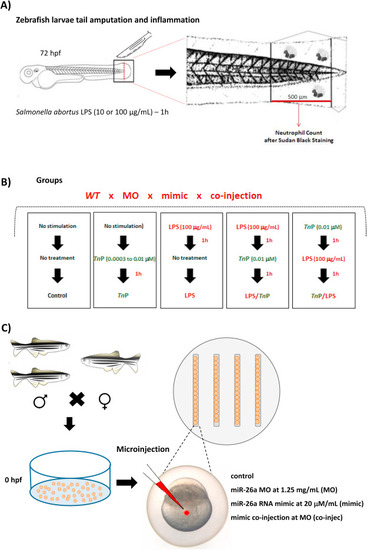
Schematic diagram of inflammation induction, TnP treatment regimens, and microinjection. Zebrafish larvae at 72 hpf previously treated with PTU (20–50 per group) with amputated tail were exposed to stimulation and treatment for 1 h each at 28 °C as described: non-stimulated and non-treated (control), non-stimulated and treated with TnP alone at 0.0003, 0.003, and 0.01 μM (TnP-group), LPS-stimulated (S. abortus at 10 or 100 μg/mL) and non-treated (LPS-group), LPS-stimulated (S. abortus at 100 μg/mL) and treated with TnP at 0.01 μM (therapeutic, LPS/TnP), or treated with TnP at 0.01 μM and LPS-stimulated (S. abortus at 100 μg/mL) (prophylactic, TnP/LPS). After regimens, anesthetized and killed larvae were fixed and stained with Sudan black for absolute neutrophil number count in 500 μm from the cut of the tail (A and B). Embryos of 0 hpf (20–50 per group) mounted in the grooves present in the agarose-coated plate were microinjected with 2 or 3 nL in the yolk with MO (at 1.25 mg/mL), mimic (20 μM/mL), or co-injected with both (MO at 1.25 mg/mL + mimic at 20 μM/mL) using a microneedle coupled to the Injectman® 4 pneumatic microinjector (C). |
|
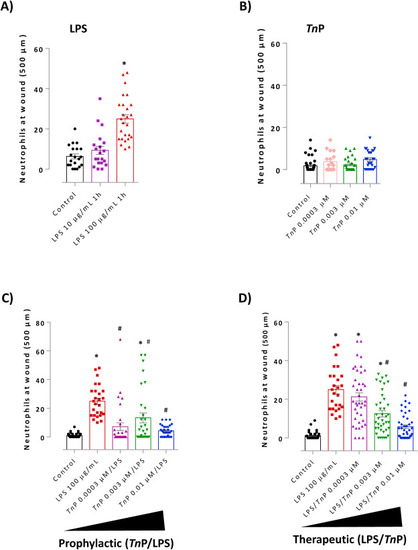
Evaluation of prophylactic and therapeutic effect of TnP on neutrophil recruitment. Zebrafish larvae at 72 hpf with amputated tail were exposed to LPS (S. abortus at 10 or 100 μg/mL - A) or TnP alone at 0.0003, 0.003, and 0.01 μM for 1 h (B). TnP at 0.0003, 0.003, and 0.01 μM were applied before (C - prophylactic, TnP/LPS) or after (D - therapeutic, LPS/TnP) LPS stimulation. Larvae with amputated caudal fins non-stimulated and non-treated were considered as control. At the end of experiments, groups of larvae were fixed and stained with Sudan black solution for the absolute neutrophil number count in 500 μm from the cut of the tail. Data represent mean ± SEM and *p < 0.01 compared with control and #p < 0.01 compared with LPS-group. |
|
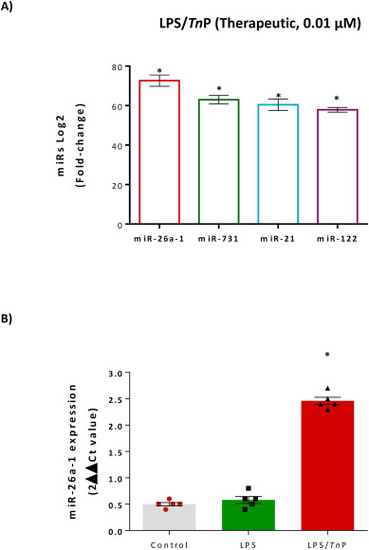
The four most highly expressed miRNAs by RNA sequencing analysis. Among the up-regulated known miRNAs, miR-26a, miR-731, miR-21, and miR-122 have the highest fold-change after 0.01 μM of TnP treatment compared to LPS-stimulated larvae (A, *p < 0.01). Expression level of miR-26a by TaqMan qPCR following administration of TnP in LPS-stimulated group versus control or LPS, normalized to reference miRNAs hsa-miR-103a-3p and hsa-miR-191-5p (B). |
|
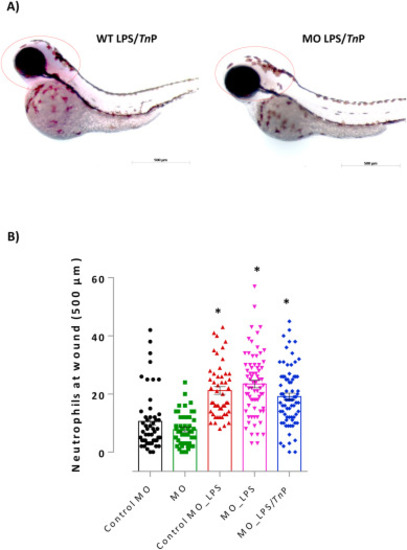
Evaluation the role of knockdown of miR-26a on anti-inflammatory activity of TnP. Lateral view of representative zebrafish larvae demonstrated strong expression of miR-26a in the head of WT LPS/TnP, but no purple precipitate was visualized in MO LPS/TnP, analyzed by miRCURY LNA miRNA digoxigenin-labeled detection probe. Dotted red circle around the head indicates positive chromogenic detection (A). Embryos were visualized on AxioVision® software (Carl Zeiss, Oberkochen, Germany) in magnification of 100×. (B) Neutrophilia to wound tail was assessed after TnP treatment of LPS-stimulated MO larvae. MO-Control or MO larvae with amputated caudal fins non-stimulated and non-treated were considered as the control. At the end of experiments, groups of larvae were fixed and stained with Sudan black solution for the absolute neutrophil number count in 500 μm from the cut of the tail. Data represent mean ± SEM and *p < 0.01 compared with MO Control-group. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.) |
|
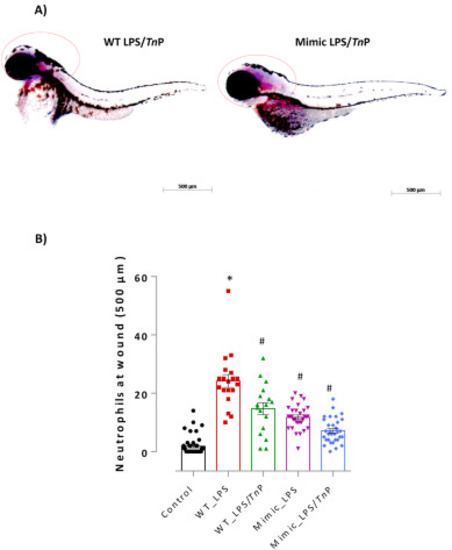
Evaluation of the role of overexpression of miR-26a on anti-inflammatory activity of TnP. Lateral view of representative zebrafish larvae demonstrated strong expression of miR-26a in the head of both groups WT LPS/TnP and Mimic LPS/TnP, analyzed by miRCURY LNA miRNA digoxigenin-labeled detection probe. Dotted red circle around the head indicates positive chromogenic detection (A). Embryos were visualized on AxioVision® software (Carl Zeiss, Oberkochen, Germany) in magnification of 100×. (B) Neutrophilia to wound tail was assessed after TnP treatment of LPS-stimulated mimic larvae. WT-Control with amputated caudal fins non-stimulated and non-treated was considered as the control. At the end of experiments, groups of larvae were fixed and stained with Sudan black solution for the absolute neutrophil number count in 500 μm from the cut of the tail. Data represent mean ± SEM and *p < 0.01 compared with WT-Control group, #p < 0.01 compared with WT LPS-group. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.) |
|
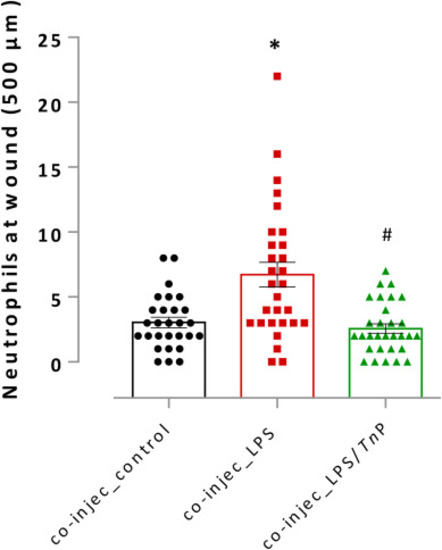
Evaluation of the co-injection on the anti-inflammatory activity of TnP. Neutrophilia to wound tail was assessed after LPS stimulation in 72 hpf co-injected larvae treated or non-treated with TnP at 0.01 μM in a therapeutic regimen. Co-injected larvae with amputated caudal fins non-stimulated and non-treated were considered as the control. At the end of experiments, groups of larvae were fixed and stained with Sudan black solution for the absolute neutrophil number count in 500 μm from the cut of the tail. Data represent mean ± SEM and *p < 0.01 compared with co-injected control-group, #p < 0.01 compared with co-injec LPS-group. |