- Title
-
Persistent Ventricle Partitioning in the Adult Zebrafish Heart
- Authors
- Pfefferli, C., Moran, H.R., Felker, A., Mosimann, C., Jaźwińska, A.
- Source
- Full text @ J Cardiovasc Dev Dis
|
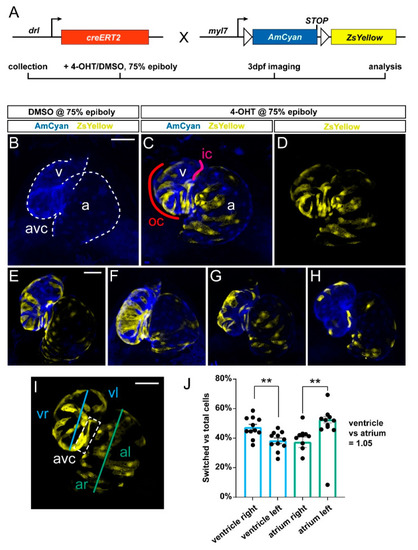
Predominant labeling of the first heart field (FHF)-assigned embryonic myocardium using drl:creERT2. (A) Crossing scheme of used transgenes. drl:creERT2 is expressed in the gastrulation-stage lateral plate mesoderm (LPM) progenitors and becomes gradually restricted to FHF descendants in the heart, while myl7:Switch is a cardiomyocyte-specific loxP reporter line that by default marks myocardium in AmCyan (blue) and ZsYellow (yellow) upon exposure to active Cre recombinase. Timeframe of involved experimental steps outlined below. See text for details. (B–H) Maximum intensity projections of confocal stacks taken from three dpf zebrafish embryos double-transgenic for dr:creERT2;myl7:Switch and 4-OH-Tamoxifen (4-OHT)- or DMSO control-induced at 75% epiboly. Ventral views, rostral to the top. Channel merge shown for a DMSO control heart ((B), note absence of ZsYellow due to absence of CreERT2 activity, n = 6) and a representative 4-OHT-treated heart together with ZsYellow lineage label only ((C,D), n = 11), atrium (a), ventricle (v), and atrio-ventricular canal (avc), color-annotated for predominantly second heart field (SHF)-derived inner curvature (ic), predominantly FHF-derived outer curvature (oc). Additional hearts of the imaging series are shown below (E–H); note the distribution of drl:creERT2-induced ZsYellow clones throughout the atrium and ventricle. (I,J) Quantification of clone distribution throughout atrium and ventricle, division lines for left vs. right atrium (al, ar) and ventricle (vr, vl), with boxed atrioventricular canal shown (I) that predominantly lies on the left side using this analysis and contributes to possible overrepresentation of left-sided clones in the ventricle. ** p = 0.002 for atrium, ** p = 0.0046 for ventricle, bar diagram depicts mean with error bars as SEM, significance based off differences was calculated using a two-tailed Student’s t-test (n = 11 hearts). Ratio of total linage labeling in ventricle versus atrium is almost identical (1.05). Scale bars: 50 µm. |
|
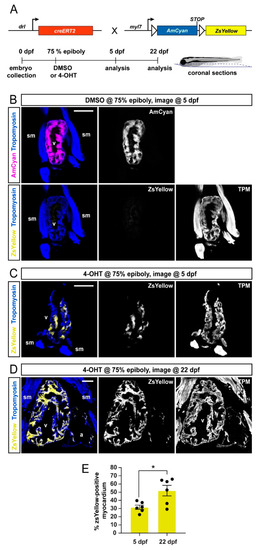
FHF lineage labeling persists during developmental stages. (A) Crossing scheme of used transgenes and timeline of experimental setup with 5 days post-fertilization (dpf) embryos and 22 dpf larvae longitudinally sectioned for heart analysis. (B–D) Confocal images of individual coronal sections, stained for pan-muscle Tropomyosin (blue, sm indicating skeletal muscles, v indicating ventricle, a indicating atrium). Embryos at 5 dpf (B), treated with DMSO (control, n = 4) at the 75% epiboly, show only myocardial AmCyan expression from myl7:Switch, while 4-OHT treatment (C) results in ZsYellow-expressing clones from drl:creERT2 activity, as also observed at 22 dpf (D). (E) Quantification of the ZsYellow–Tropomyosin double-positive cardiomyocytes of analyzed ventricles, two-tailed Student’s t-test, * p = 0.0149, 5 dpf n = 6; 22 dpf n = 6. Scale bar 50 µm. |
|
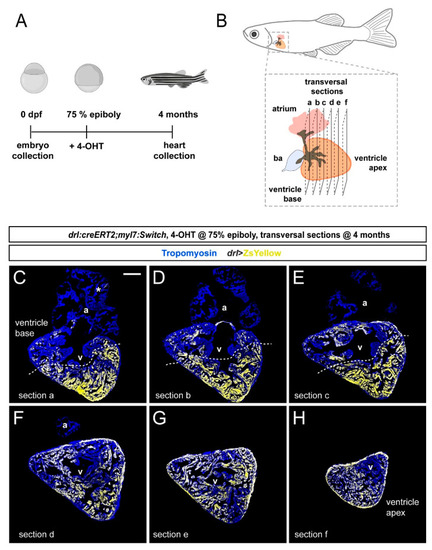
FHF contribution to the adult zebrafish ventricle remains localized. (A) Schematic of the experimental design and timeline. Embryos were 4-OHT-treated at 75% epiboly and raised to adulthood for 4 months. (B) Hearts were dissected from the adult animals and sequentially sectioned from the base of the heart to the apex as per schematic. (C–H) Series of transversal sections of a selected heart, immunostained against Tropomyosin (blue) and showing ZsYellow expression (yellow) from drl:creERT2-recombined myl7:Switch with atrium (a) on top and ventricle (v) at the bottom. Asterisk denotes rare atrial ZsYellow clones throughout sections. Note the markedly larger area of ZsYellow-labeled cardiomyocytes in the ventricle. Scale bar: 200 µm. |
|
Quantification of localized FHF lineage labeling in the adult zebrafish ventricle. (A) Schematic illustration of the adult zebrafish heart with three planes of transversal sections across the region of the ventricle base and the atrium. (B) Quantification of ZsYellow-positive cardiomyocyte area in the Tropomyosin-demarcated myocardium indicates predominant labeling in the atrium–distal ventricle, while the atrium–proximal ventricle only retained low numbers of ZsYellow-labeled cells. Note the scarce atrium labeling. One-Way ANOVA test followed by Tukey’s multiple comparison test, *** p < 0.0001 (n = 8 hearts, 2–3 sections each as per (A)). (C–E) Three sections (a, b, c) of the same heart with the largest tissue area were selected for the quantification of ZsYellow-labeled cardiomyocytes. The dotted line depicts a possible boundary of high- versus low-density ZsYellow labeling. Note that nonetheless, no sharp boundary separates the two areas. Asterisk denotes rare atrial ZsYellow clones throughout sections. Scale bar: 200 µm. |
|
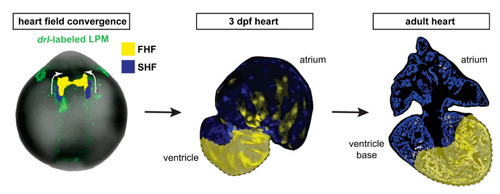
draculin (drl)-based FHF lineage tracing of cardiomyocytes reveals compartmentalization of the adult zebrafish ventricle. Model summary of the reported findings. FHF and SHF descendants in the anterior LPM (ALPM) form the early-differentiating and late-differentiating myocardium, respectively, in the early heart. Then, in the adult heart, the FHF myocardium predominantly contributes to the atrium-distal portion of the ventricle including the apex, whereas the atrium-proximal region of the ventricle base is predominantly SHF-derived. Nonetheless, the interface between the FHF and SHF regions is not a sharp compartment boundary. |