- Title
-
5-Bromoprotocatechualdehyde Combats against Palmitate Toxicity by Inhibiting Parkin Degradation and Reducing ROS-Induced Mitochondrial Damage in Pancreatic β-Cells
- Authors
- Cha, S.H., Zhang, C., Heo, S.J., Jun, H.S.
- Source
- Full text @ Antioxidants (Basel)
|
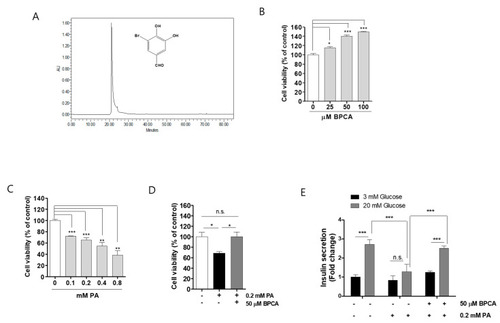
5-Bromoprotocatechualdehyde (BPCA) isolated from Polysiphonia japonica combats palmitate-induced toxicity and dysfunction in Ins-1 cells. (A). Chemical structure and high-performance liquid chromatography (HPLC) purity profile of BPCA. (B). Ins-1 cells were incubated with the indicated concentrations of BPCA (25, 50, and 100 µM) for 24 h. (C). Ins-1 cells were incubated with the indicated concentrations of palmitic acid (PA) (0.1, 0.2, 0.4, and 0.8 mM) for 24 h. (D). Ins-1 cells were incubated with 50 µM BPCA for 1 h and then further incubated with/without 0.2 mM PA for 24 h. CCK-8 assays were subsequently performed as described in “Materials and Methods”. (E). Ins-1 cells were incubated with 50 µM BPCA in 5 mM glucose media for 1 h and then further incubated with or without 0.2 mM PA for 24 h. Thereafter, the cells were starved in 0.2 mM glucose-containing Krebs–Ringer bicarbonate (KRB) buffer for 2 h. Insulin release was measured after 2 h of incubation in either 3 or 20 mM glucose. ELISA assays for insulin were subsequently performed. Data are expressed as the fold change from untreated cells in 3 mM glucose. * p < 0.05, ** p < 0.01, *** p < 0.001, n.s. = no significance. |
|
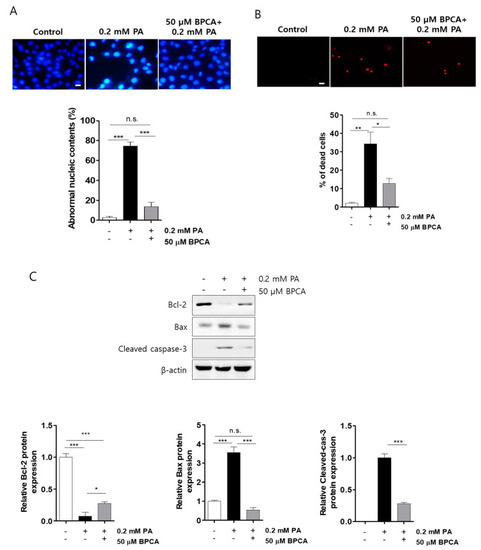
5-Bromoprotocatechualdehyde (BPCA) isolated from Polysiphonia japonica protects PA-induced cell death in Ins-1 cells. Ins-1 cells were incubated with 50 µM BPCA for 1 h and then further incubated with/without 0.2 mM PA for 24 h. (A) Further incubation with Hoechst 33342 (HO342, 10 µg/mL) subsequently performed in the images. (B) Further incubation with propidium iodide (PI, 50 µg/mL) subsequently performed in the images. Scale bar: 10 µm. (C). Western blotting was subsequently performed as described in “Materials and Methods”. * p < 0.05, ** p < 0.01, *** p < 0.001, n.s. = no significance. |
|
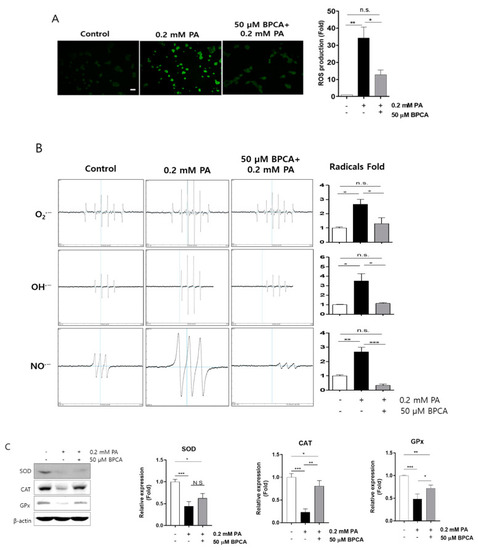
5-Bromoprotocatechualdehyde (BPCA) isolated from Polysiphonia japonica prevents PA-induced reactive oxygen species (ROS) overproduction by preserving antioxidant enzymes in Ins-1 cells. Ins-1 cells were incubated with 50 µM BPCA for 1 h, and then further incubated with/without 0.2 mM PA for 12 h. (A) Intracellular ROS was subsequently measured using 10 µM 2,7-dichlorofluorescein diacetate (DCFH-DA). Scale bar: 10 µm. (B) Radical production was subsequently determined using an electron spin resonance (ESR) spectrometer as described in “Materials and Methods”. (C) Western blotting was subsequently performed as described in “Materials and Methods”. * p < 0.05, ** p < 0.01, *** p < 0.001, n.s. = no significance. |
|
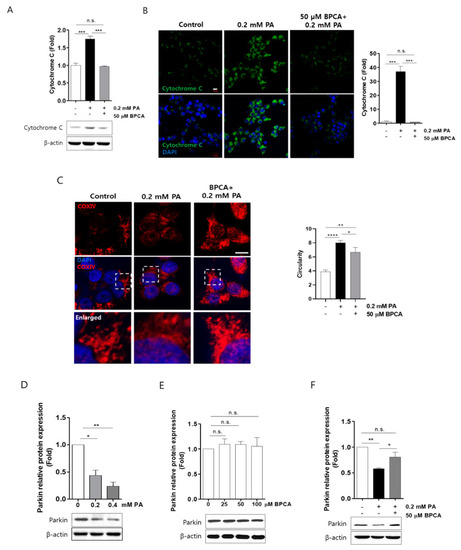
5-Bromoprotocatechualdehyde (BPCA) isolated from Polysiphonia japonica protects PA-induced degradation of parkin by combating cytochrome C release and morphology alteration of mitochondria in Ins-1 cells. Ins-1 cells were incubated with 50 µM BPCA for 1 h and then further incubated with/without 0.2 mM PA for 24 h. (A) Western blotting was subsequently performed as described in “Materials and Methods”. (B,C) Confocal image was subsequently performed as described in “Materials and Methods”. Scale bar: 10 µm. Circularity measures the average value per high-resolution micrograph using ImageJ. (D) Ins-1 cells were incubated with the indicated concentrations of PA (0.2 and 0.4 mM) for 24 h. (E) Ins-1 cells were incubated with the indicated concentrations of BPCA (25, 50, and 100 µM) for 24 h. (F) Ins-1 cells were incubated with 50 µM BPCA for 1 h and then further incubated with/without 0.2 mM PA for 24 h. Western blotting was subsequently performed as described in “Materials and Methods”. * p < 0.05, ** p < 0.01, *** p<0.005, **** p<0.001, n.s. = no significance. |
|
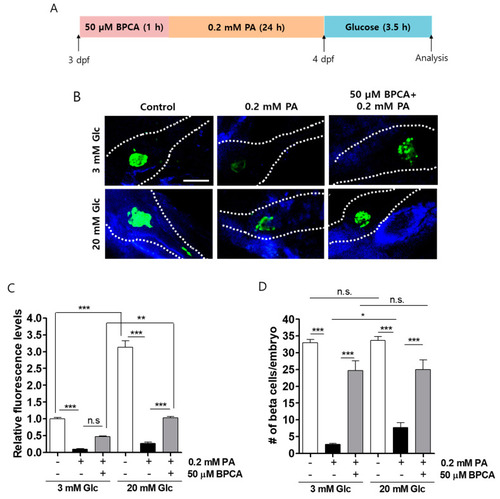
5-Bromoprotocatechualdehyde (BPCA) isolated from Polysiphonia japonica protects against PA-induced β-cell dysfunction in zebrafish. (A) Zebrafish were incubated with 50 µM BPCA and 0.2 mM PA from 3 to 4 days post-fertilization (dpf). BPCA was added 1 h prior to PA treatment. Thereafter, the zebrafish were incubated with 3 or 20 mM glucose for 3.5 h. (B) Confocal microscopy images of the pancreata of the zebrafish. Scale bar: 100 µm. (C). Relative EGFP fluorescence levels from (B). (D) Numbers of β-cells per embryo from (B). n = 4~6. * p < 0.05, ** p < 0.005, *** p < 0.001, n.s. = no significance. |