- Title
-
Induction of Short-Term Sensitization by an Aversive Chemical Stimulus in Zebrafish Larvae
- Authors
- Roberts, A.C., Alzagatiti, J.B., Ly, D.T., Chornak, J.M., Ma, Y., Razee, A., Zavradyan, G., Khan, U., Lewis, J., Natarajan, A., Baibussinov, A., Emtage, J., Komaranchath, M., Richards, J., Hoang, M., Alipio, J., Laurent, E., Kumar, A., Campbell, C.S., Stark, R., Carmona, J., Hussain, A., Scaramella, C., Husain, J., Buck, R., Jafarpour, A., Garcia, M., Mendoza, S., Sandoval, G., Agundez, B., Fink, A., Osadi, F., Deutsch, E., Hernandez, S.C., Arisaka, K., Glanzman, D.L.
- Source
- Full text @ eNeuro
|
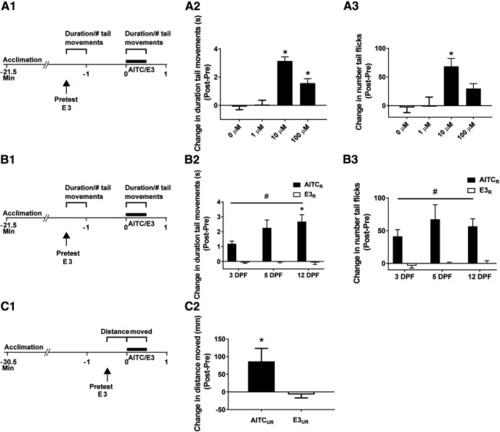
Locomotor activity in zebrafish larvae is enhanced in the presence of AITC. |
|
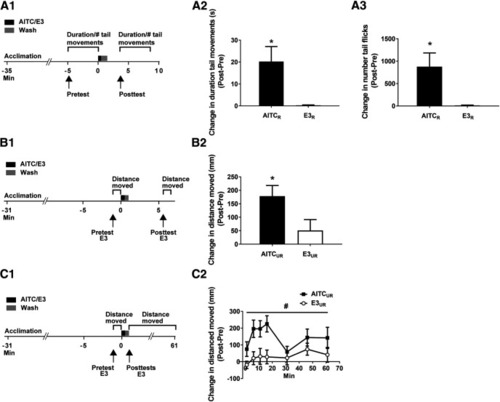
AITC elicits persistently enhanced locomotion in zebrafish larvae. |
|
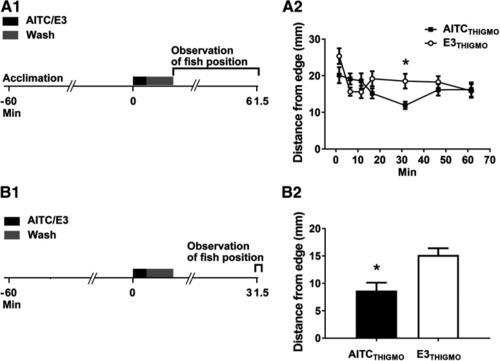
Thigmotaxis is enhanced by AITC in larval zebrafish. |
|
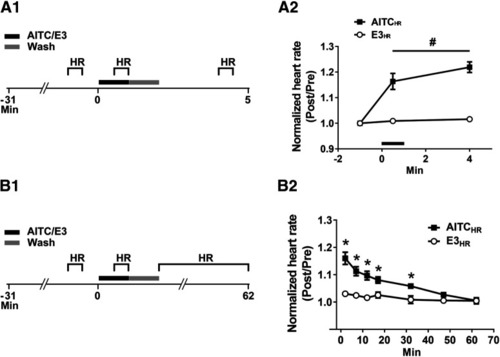
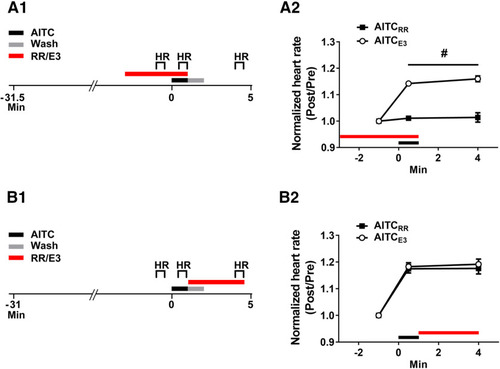
Heart rate in larval zebrafish is persistently increased by AITC. |
|
An antagonist of TRP channels blocks the increase in larval heart rate observed during the presence of AITC, but not that observed after washout of the irritant. |
|
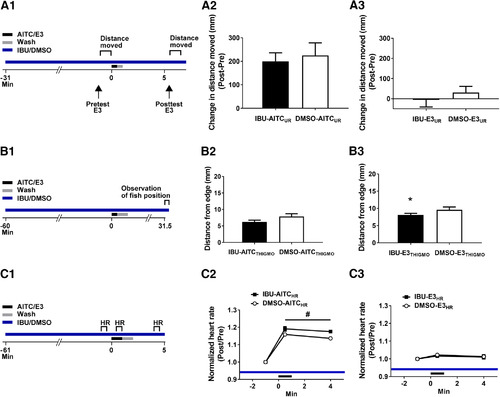
IBU does not block the enhancement of locomotion, thigmotaxis, and heart rate caused by AITC in larval zebrafish. |
|
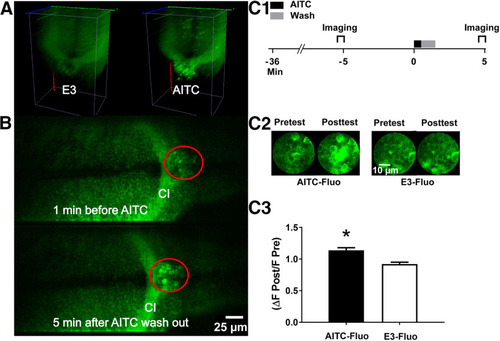
AITC causes an increase in neuronal activity that persists after washout in a hindbrain region of the larval brain. |