- Title
-
Paclitaxel-induced peripheral neuropathy is caused by epidermal ROS and mitochondrial damage through conserved MMP-13 activation
- Authors
- Cirrincione, A.M., Pellegrini, A.D., Dominy, J.R., Benjamin, M.E., Utkina-Sosunova, I., Lotti, F., Jergova, S., Sagen, J., Rieger, S.
- Source
- Full text @ Sci. Rep.
|
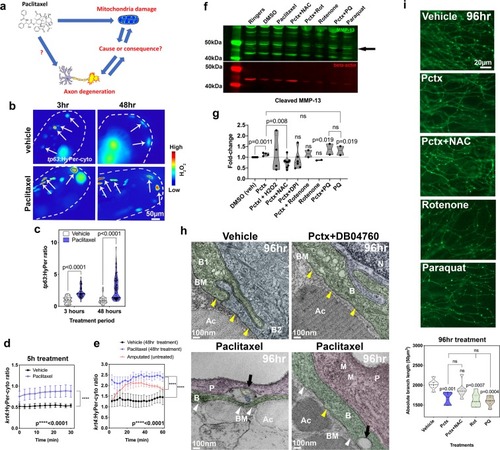
Mitochondrial ROS contribute to MMP-13 expression and axon degeneration. ( EXPRESSION / LABELING:
PHENOTYPE:
|
|
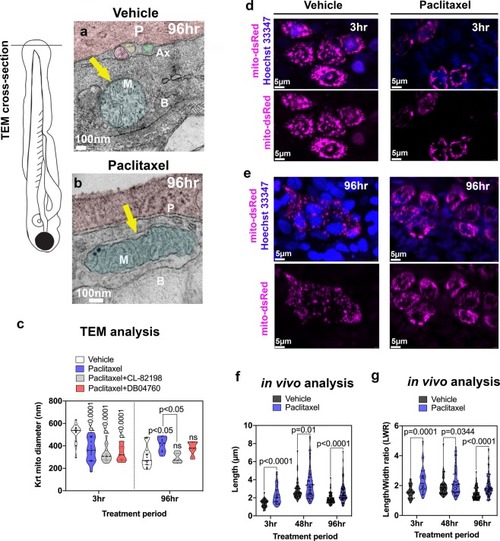
Keratinocyte mitochondria are damaged by paclitaxel treatment. ( |
|
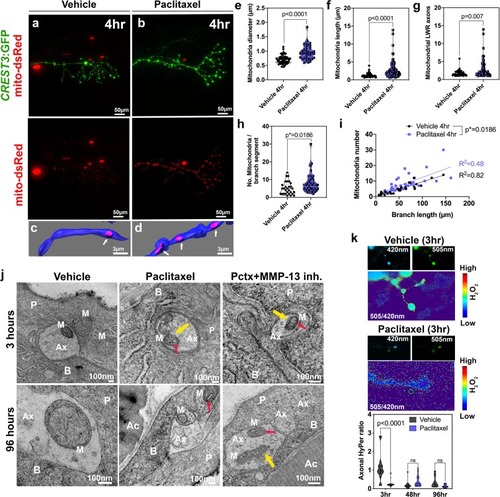
Axonal mitochondria are vacuolized following paclitaxel treatment but ROS/H2O2 levels are not elevated. ( |
|
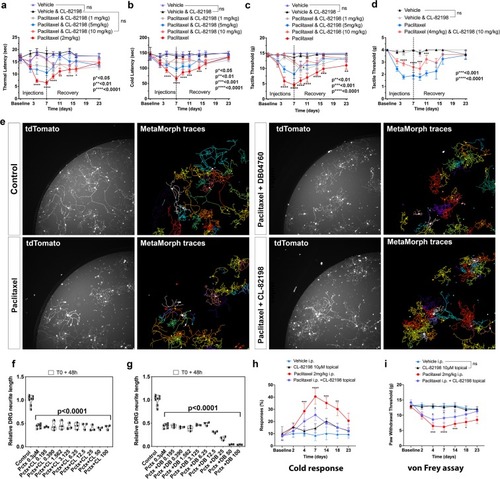
MMP-13 prevents paclitaxel-induced PIPN in rodents by neuron-extrinsic mechanism. ( |