- Title
-
Visualizing trypanosomes in a vertebrate host reveals novel swimming behaviours, adaptations and attachment mechanisms
- Authors
- Dóró, É., Jacobs, S.H., Hammond, F.R., Schipper, H., Pieters, R.P., Carrington, M., Wiegertjes, G.F., Forlenza, M.
- Source
- Full text @ Elife
|
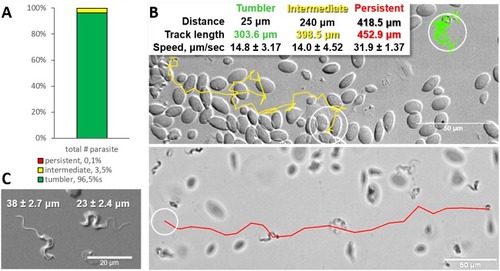
Majority of trypanosomes in freshly drawn blood are tumblers. Blood was freshly drawn from carp and T. carassii swimming behaviour analysed immediately using high-resolution microscopy at 240 frames per second (fps). (A) Relative percentage of tumblers, and intermediate or persistent swimmers (defined in the text) was calculated over a total number of 944 T. carassii, isolated from six different carp infections and imaged over 60 independent acquisitions. (B) Representative tracks of a tumbler (green), intermediate (yellow) and persistent (red) swimmer. The diameter of the circles (23 µm) indicates the average cell-body size of a trypanosome as also shown in (C). The inset table summarizes the straight-line distance covered by the trypanosome (between the first and last track point); the total track length, that is the path covered by the trypanosome in approximately 20 s of acquisition time, indicated in matching colours; and the average speed (μm/s) was calculated on a selection of the acquisitions used in (A). For tumblers, the displacement of the posterior end was used as tracking point. (C) Detailed image of two trypanosomes indicating the total body length including the flagellum (left) and the total cell-body length excluding the flagellum (right). Measurements were acquired on high-resolution images of at least 10 freshly isolated trypanosomes obtained from four independent infections, using more than 20 frames within the same acquisition. Quantification of trypanosome length, swimming speed and directionality was performed with ImageJ-Fijii using the MTrack plug-in. Video 1 displays high-speed videos of the swimming behaviour of tumblers, intermediate and persistent swimmers in carp blood, or of trypanosomes in serum or culture medium. |
|
( |
|
( |
|
( |
|
Zebrafish larvae (5 dpf) were infected with 200 PHENOTYPE:
|
|
Wild type zebrafish larvae (5 dpf) were infected with 200 PHENOTYPE:
|
|
( |