- Title
-
Signaling Events Downstream of AHR Activation That Contribute to Toxic Responses: The Functional Role of an AHR-Dependent Long Noncoding RNA ( slincR) Using the Zebrafish Model
- Authors
- Garcia, G.R., Shankar, P., Dunham, C.L., Garcia, A., La Du, J.K., Truong, L., Tilton, S.C., Tanguay, R.L.
- Source
- Full text @ Environ. Health Perspect.

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. |
|
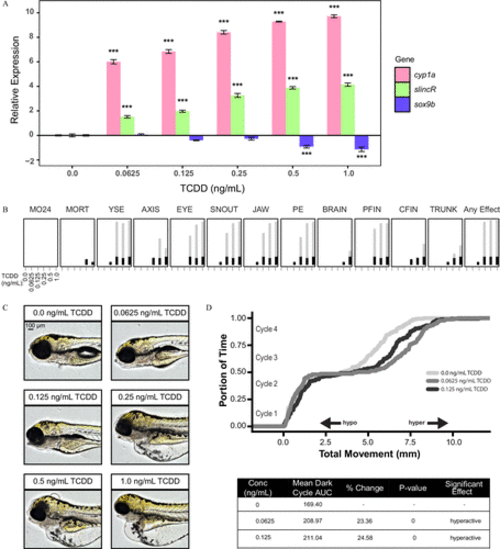
Analysis of the concentration–response effects of developmental exposure (1-h exposure at 6hpf ) to TCDD on gene expression at 48hpf and morphological malformations at 120hpf . (A) qRT-PCR relative expression of cyp1a, slincR, and sox9b transcripts in 48-hpf wild-type embryos exposed to 0, 0.625, 0.125, 0.25, 0.5, or 1.0ng/mL TCDD. For all assays, 0.1% DMSO served as the vehicle control and is listed as 0ng/mL TCDD. Expression values were analyzed with the 2−ΔΔCT method and normalized to β-actin using the 0ng/mL TCDD concentration as the calibrator. Each experimental unit represents a pool of 20 embryos, and each treatment group included four biological replicates ( n=4 ). The data were log2-transformed and analyzed using a one-way ANOVA with a Dunnett post hoc test ( p<0.001 in comparison with 0ng/mLTCDD=*** ). All error bars indicate standard error of the mean. (B) Evaluation of 17 physical malformations at 120-hpf on wild-type zebrafish exposed to six concentrations of TCDD ( 0–1ng/mL ) across two 96-well plates. Nonsignificant malformations (otic vesicle, somite, circulation, pigmentation, swim bladder, notochord distortion, and alterations in touch response) are excluded. The horizontal axis displays the TCDD concentrations tested, and the malformation examined is listed above each box. The incidence across all replicates is plotted as stacked points. For each malformation, the stacked points exceeding the binomial significance threshold are represented in light gray (top stack). The data were analyzed using a Fisher’s exact test with a Bonferroni correction for multiple comparisons ( n=32 , p<0.01 ). (C) Representative lateral images of 120-hpf zebrafish for each concentration of TCDD tested. The bar in top left corner indicates 100μM . (D) Larval photomotor response (LPR) in 120-hpf wild-type embryos developmentally exposed to 0, 0.0625, or 0.125ng/mL TCDD equally divided across two 96-well plates using the ViewPoint ZebraBox larvae screening system. For each concentration of TCDD, the overall area under the curve was analyzed for the last 3 light:dark cycles in comparison with 0ng/mL TCDD using a Kolmogorov-Smirnov test ( n=32 , p≤0.01 ). |
|
Effect of 1ng/mL TCDD on the developing jaw after treatment of control and slincR morphants. Control (Con-MO) and slincR morphants (slincR-MO) were developmentally exposed to 0.1% DMSO or 1ng/mL TCDD, and the cartilage was stained and measured at 72hpf . (A) A morphometric system was used to measure the position and length of landmark structures in the developing jaw. The position of jaw structures representing junctions between Meckel’s and palatoquadrate cartilages (point A and B) and representing junctions between the hyosymplectic and ceratohyal cartilages (point C and D) was measured relative to a reference point as shown. Statistical significance was determined by a modified two-way ANOVA with a Tukey post hoc test. Morphometric values represent mean±standarderrorofthemean . ( n=9–10 individual 72-hpf zebrafish; p<0.05=* , p<0.01=** , p<0.001=*** ). The asterisk (*) indicates statistical significance between slincR-MO and Con-MO samples exposed to TCDD. (B) Representative images of the cartilage structures in control and slincR morphants treated with DMSO or TCDD. The arrow in the TCDD Trace panel points to the junction between the hyosymplectic and ceratohyal cartilages (point C and D). The bar in the top right corner indicates 100μM . PHENOTYPE:
|
|
TCDD-induced hemorrhaging in control and slincR morphants. Control (Con-MO) and slincR morphants (slincR-MO) were developmentally exposed to 0.1% DMSO or 1ng/mL TCDD, and 48-hpf zebrafish embryos were evaluated for the presence or absence of hemorrhaging. (A) The percent incidence of the hemorrhaging phenotype in control and slincR morphants exposed to DMSO or TCDD. The DMSO and TCDD samples consisted of 3 and 5 biological replicates, respectively. Each biological replicate contained 10–12 individual 48-hpf zebrafish. All error bars indicate standard error of the mean. Statistical significance was determined using the Type III one-way ANOVA for unbalanced experimental designs from the car package with a Tukey post hoc test ( n=3 or 5, p<0.001=*** ). (B) Representative images of 48-hpf wild-type zebrafish exposed to DMSO (normal phenotype) or TCDD (hemorrhaging phenotype). The bar in the top right corner indicates 100μM and arrows point to sites of hemorrhaging. PHENOTYPE:
|