- Title
-
Developmental protein kinase C hyper-activation results in microcephaly and behavioral abnormalities in zebrafish
- Authors
- Liu, T., Shi, Y., Chan, M.T.V., Peng, G., Zhang, Q., Sun, X., Zhu, Z., Xie, Y., Sham, K.W.Y., Li, J., Liu, X., Ho, I.H.T., Gin, T., Lu, Z., Wu, W.K.K., Cheng, C.H.K.
- Source
- Full text @ Transl Psychiatry
|
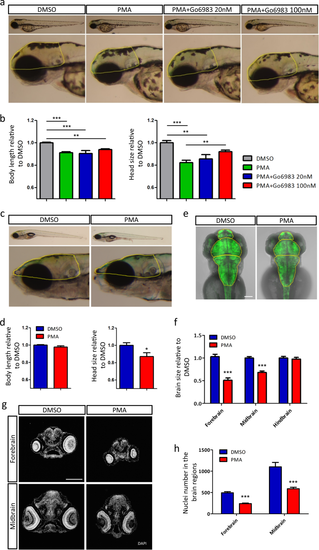
PKC overactivation during early development causes mild developmental delay and brain size deficit. a Lateral views of representative larvae (3 dpf) exposed to DMSO, PMA or PMA+Go6983. Head size was calculated with the boundary shown in yellow dotted line. b Quantitative representation of body length and head size (3 dpf) normalized to DMSO (DMSO n = 8, PMA n = 10, PMA+Go6983 20 nM n = 6, PMA+Go6983 100 nM n = 10, **p < 0.01, ***p < 0.001, one-way ANOVA). c Lateral views of representative larvae (9 dpf) previously exposed to DMSO or PMA. Head size was calculated with the boundary shown in yellow dotted line. d Quantitative representation of body length and head size (9 dpf) normalized to DMSO (DMSO n = 8, PMA n = 6, *p < 0.05, t-test analysis). e Dorsal views of zebrafish brains (3 dpf) visualized by Huc:GFP. Brain region was divided by yellow dotted line. Scale bar, 100 μm. f Quantitative representation of different brain regions normalized to DMSO (n = 16 per group, ***p < 0.001, t-test analysis). g DAPI staining of zebrafish head sections (3 dpf). Scale bar, 100 μm. h Nuclei number in forebrain and midbrain (n = 6 per group, ***p < 0.001, t-test) EXPRESSION / LABELING:
PHENOTYPE:
|
|
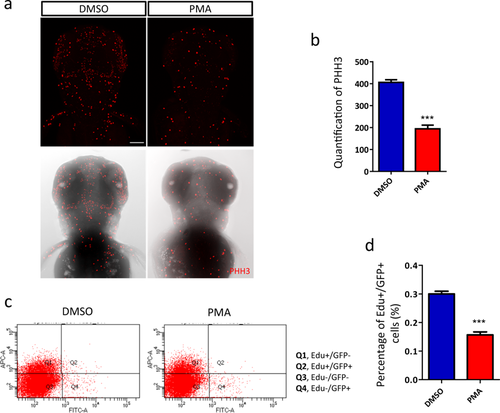
Aberrant PKC activation impaired neurogenesis. a, b Reduced mitotic cells in zebrafish head (3 dpf). a Fluorescence of pHH3-positive dots (the upper row) merged with brightfield images of zebrafish head (the lower row) (n = 26 per group, ***p < 0.001, t-test). Scale bar, 100 μm. c Flow cytometry analysis of the different populations of cells. d Newborn neurons (Q2) are significantly reduced in PMA-exposed fish (n = 9 per group, p < 0.001, t-test) EXPRESSION / LABELING:
PHENOTYPE:
|
|
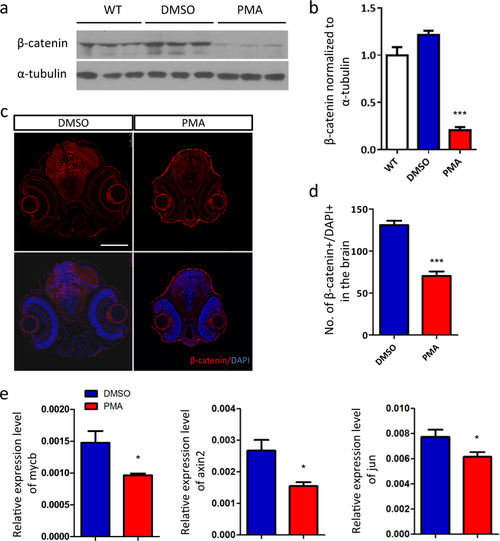
Excessive PKC signaling promotes the degradation of β-catenin. a Western blot analysis of β-catenin. b Quantification of the protein level of β-catenin relative to wild-type (WT) group (n = 3 per group, ***p < 0.001, t-test). c The nuclear accumulation of β-catenin is reduced by PMA treatment, d indicated by the quantification of β-catenin+/DAPI+ dots in brain region (n = 6 per group, ***p < 0.001, t-test). e RT-qPCR analysis of the transcriptional target of β-catenin, mycb, axin2 and jun (n = 6 per group, *p < 0.05, t-test) EXPRESSION / LABELING:
PHENOTYPE:
|
|
Stabilizing of β-catenin by gsk3β inhibition partially restores the head size deficit. a Western blot analysis of β-catenin. b Lateral views of representative larvae (3 dpf) exposed to DMSO, PMA or PMA+LiCl. Head size was calculated with the boundary shown in yellow dotted line. c Quantitative representation of body length and head size (3 dpf) normalized to DMSO (DMSO n = 14, PMA n = 18, PMA+LiCl 50 mM n = 12, PMA+LiCl 100 mM n = 18, *p < 0.05, ***p < 0.001, one-way ANOVA) EXPRESSION / LABELING:
PHENOTYPE:
|

ZFIN is incorporating published figure images and captions as part of an ongoing project. Figures from some publications have not yet been curated, or are not available for display because of copyright restrictions. PHENOTYPE:
|
|
PKC over-activation induce a transient increase of cell apoptosis within the head. (a) TUNEL staining of zebrafish head. (b) Quantification of TUNEL signaling. (* p<0.05, t-test). |
|
PKC hyper-activation has a mild effect on neurogenesis at 24hpf (a) pHH3 and TUNEL staining of zebrafish embryos. (b) Quantification of pHH3 and TUNEL staining within zebrafish head. (* p<0.05, t-test). |