- Title
-
Dynamic changes in microglial and macrophage characteristics during degeneration and regeneration of the zebrafish retina
- Authors
- Mitchell, D.M., Lovel, A.G., Stenkamp, D.L.
- Source
- Full text @ J Neuroinflammation
|
Ouabain-induced retinal degeneration results in a robust accumulation of responding immune cells. Images show cryosections from undamaged (control, |
|
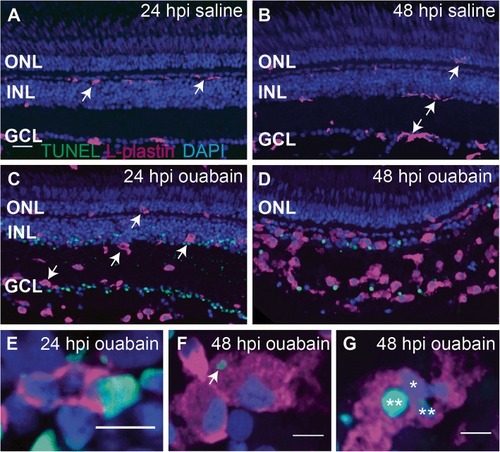
Progression of ouabain-induced retinal cell death and accumulation of immune cells in damaged retinas. Cryosections (5 μm thick) from retinas at 24 and 48 h post-intravitreal injection (24 and 48 hpi) of saline ( |
|
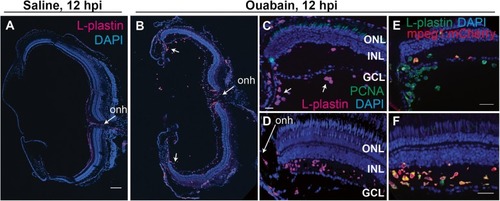
Evidence of early infiltration of immune cells to the retina at 12 h post-ouabain injection. Images of retinal cryosections at 12 h post-saline ( |
|
Distribution and proliferation markers in immune cells during the response to retinal damage. Images show staining of L-plastin (magenta), PCNA (green), and DAPI (blue) in cryosections of retinas injected with saline ( |
|
Progression of retinal degeneration and immune cell response visualized by Hematoxylin & Eosin (H&E). H&E staining of cryosections to visualize progression of the ouabain-induced lesion and immune cell accumulation. |
|
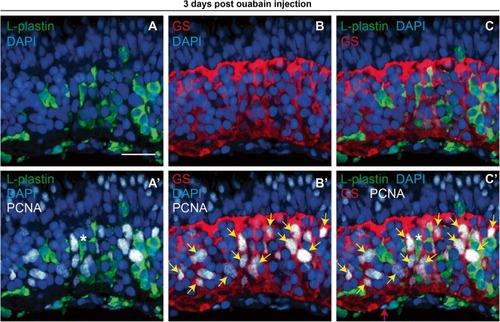
PCNA expression in immune cells and Müller glia at 72 h post-ouabain injection. Representative images of retinal cryosections at 72 h post-ouabain injection (72 hpi) stained for L-plastin to mark immune cells (green), glutamine synthetase to mark Müller glia (GS, red), PCNA (white), and DAPI (blue). |
|
Immune cells responding to ouabain-induced retinal degeneration identify as macrophages. |
|
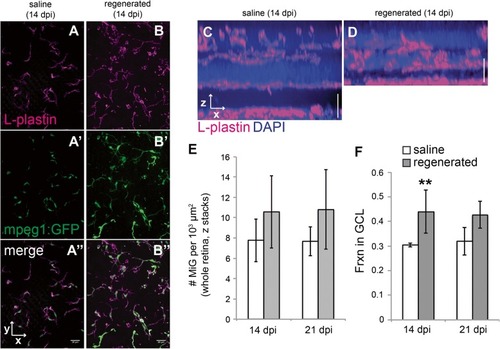
Microglia distribute to regions containing histologically regenerated retinal neurons. |
|
Morphological features of microglia in ganglion cell layer of histologically regenerated retinas. |