- Title
-
Imaging of viral neuroinvasion in the zebrafish reveals that Sindbis and chikungunya viruses favour different entry routes
- Authors
- Passoni, G., Langevin, C., Palha, N., Mounce, B.C., Briolat, V., Affaticati, P., De Job, E., Joly, J.S., Vignuzzi, M., Saleh, M.C., Herbomel, P., Boudinot, P., Levraud, J.P.
- Source
- Full text @ Dis. Model. Mech.
|
SINV replicates in zebrafish larvae and exhibits a broad organ tropism. (A) Scheme of a 72 hours post-fertilization (hpf) larva, showing the sites of injection: IV, intravenously in the caudal vein (CV) or the dorsal aorta (DA); IC, intracerebrally in the optic tectum; eye, in the retina. (B) Virus replication in IV-infected zebrafish larvae, assessed by titration of homogenates of whole larvae. Data represent the means±s.e.m. of five individual larvae per time point, from three experiments pooled. (C) Survival curves of control uninfected (No V) and IV-infected zebrafish larvae (SINV). Data were pooled from five independent experiments. n=60 larvae per group. (D) Live detection of SINV-infected cells by in vivo confocal imaging, with superposition of transmitted light and GFP fluorescence (maximal projection). (D′) Uninfected control (No V), 4 dpf (1 dpi). (D″,D‴) The same IV SINV-infected (SINV) larva at 1 dpi (D″) and 2 dpi (D‴). H, heart (dotted red line); L, liver (dotted yellow line); Y, yolk (note that the yolk is autofluorescent but, as shown in D′, at a nearly undetectable level using these imaging conditions; the signal in D″ corresponds to infection); white arrowhead, infection in the left pectoral muscle; blue arrowhead, infection in the swim bladder; orange arrowhead, infection in the brain. Scale bars: 50 μm. In this and all following lateral view figures, anterior is to the left and dorsal to the top. |
|
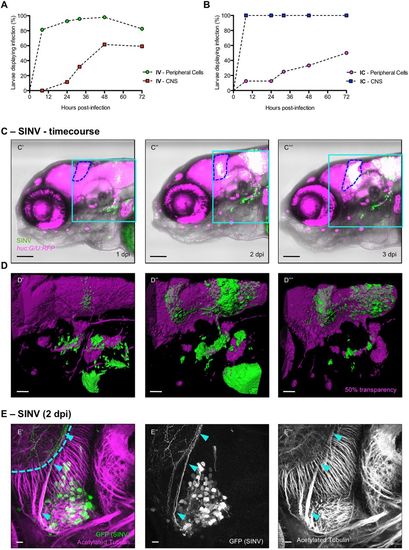
SINV is neuroinvasive in zebrafish larvae. (A,B) Quantification of the appearance of infected cells in the periphery (circles) and in the CNS (squares), from in vivo observation of huC:G/U:RFP larvae, following IV (A) or IC (B) inoculation. n=12-24 from two independent experiments pooled. (C′-C‴) Live confocal imaging of the same IV SINV-infected huc:G/U:RFP larva from 1 to 3 dpi, showing the progression of the infection. Superposition of transmitted light, green (infected cells) and red (neurons) fluorescence; blue dotted line, cerebellum. In this and following colour figures, red fluorescence is displayed in magenta and referred to as ‘magenta fluorescence’. The light blue squares correspond to regions shown in panels D′-D‴. Scale bars: 50 µm. (D′-D‴) 3D rendering of the brain areas shown above. Scale bars: 25 µm. (E′-E‴) Confocal image of a whole-mount immunohistochemistry processed SINV-infected WT larva, maximal projection. GFP staining (SINV-infected cells) in green; acetylated tubulin (axons) in magenta. Infected cells are in the trigeminal ganglion (TG); the axon-rich region visible behind (light blue dotted line) is the neuropile of the left optic tectum (OT). Light blue arrowheads point to the axon of an infected neuron connecting the TG to the OT. Scale bars: 10 µm. |
|
CHIKV, but not SINV, infects the brain microvascular endothelium. Dorsal views of fli:G/U:RFP larvae, with infected cells in green and endothelial cells shown in magenta; confocal imaging, maximal projections. For all panels, the top image (with ′) shows superposition of transmitted light with green and magenta fluorescence, and the bottom image (with ″) shows only the magenta fluorescence to provide better visualization of the vasculature. Scale bars: 50 μm. (A,B) Live imaging of the same SINV-infected larva at 1 dpi (A) and 2 dpi (B). See also Fig. S1A, Movies 1 and 2, for a view through the z-stack. (C) Live imaging of control uninfected (No V) larva. (D,E) Fixed CHIKV-infected larvae at 1 dpi (D) and 2 dpi (E). See also Fig. S1B and Movie 4, for a view through the z-stack. (F) Fixed IHNV-infected larva at 2 dpi. See also Movie 5, for a view through the z-stack. In this and all following dorsal view figures, anterior is to the left. |
|
SINV does not induce BBB leakage. In vivo assay for BBB permeability. Uninfected controls, IHNV- and SINV-infected fliG/U:RFP larvae were injected IV at 1 and 2 dpi with 10 kDa FITC-dextran. One hour later, live confocal imaging of the brain was performed, to compare FITC intensity within the CBV and BP. (Injections at 2 dpi were performed only on uninfected controls and SINV-infected larvae, because of early death of IHNV-infected larvae.) (A,B) Representative images of IHNV-infected (A) and SINV-infected (B) larvae; single confocal plane at same depth. Scale bars: 100 μm. (C) Ratios of FITC fluorescence intensities measured in BP and CBV. n=2-5 larvae per group; two separate focal planes per larva. |
|
Macrophages are not infected by SINV or CHIKV and not required for neuroinvasion. (A,B) Whole-mount immunohistochemistry of SINV-infected (A) or CHIKV-infected (B) mpeg:G/U:Nfsb-mCherry (macrophages in magenta) larva at 2 dpi. Lateral views of the head; confocal imaging, maximal projection. (A′,B′) Merge of transmitted light with green (infected cells) and magenta (macrophages) fluorescence. (A″,B″) Green fluorescence showing infected cells. (A‴,B‴) Magenta fluorescence showing macrophage distribution. (A‴′,B‴′) Magnification of the area boxed in A′ and B′; green and magenta fluorescence, 3D rendering slightly tilted. Scale bars: 50 µm (A′-A‴,B′-B‴), 25 µm (A″″,B″″). (C) Macrophage depletion in mpeg:G/U:Nfsb-mCherry larvae. Superposition of transmitted light and magenta (macrophage) fluorescence, maximal projection, lateral view of the head, in a DMSO-treated control (top) or 2 days after treatment with metronidazole (bottom). (D) Impact of macrophage depletion on occurrence of neuroinvasion. mpeg:G/U:Nfsb-mCherry larvae were treated with DMSO or metronidazole, before injection with SINV (top graph) or CHIKV (bottom graph). Percentage of brain-infected larvae. n=36 from three independent experiments pooled. ns, not significant (Log-rank test). |
|
Efficient axonal transport of SINV. (A) Infection of muscle cells and connected spinal cord neurons in the tail region of a huc:G/U:RFP larva. Live confocal imaging, maximal projection, 3D rendering, with superposition of green (infected cells) and magenta (neurons) fluorescence. Same larva imaged at 1 (A′), 2 (A″) and 3 dpi (A‴). Dotted white lines indicate the limits of the fins; dash-dotted lines the limits of the spinal cord. Scale bars: 50 µm. (B,C) Assay for axonal transport to the contralateral optic tectum after inoculation of SINV (B) or CHIKV (C) in the left retina. Confocal imaging of fixed infected larva at 2 dpi, with superposition of green (infected cells) and magenta (acetylated tubulin) fluorescence; maximal projections. Scale bars: 50 μm. (B′) Scheme of the projection of the retinal neurons to the optic tectum. (D) Ratio of GFP fluorescence intensities measured in the eye and the contralateral optic tectum, after SINV or CHIKV inoculation. n=5 from two independent experiments pooled. |
|
High magnification imaging of vasculature in infected larvae. Confocal imaging, single focal planes extracted from Fig. (3A') and (3D'). Scale bars: 50 μm. (A) SINV infection live imaging, from Fig. (3A'); (A') merge of green (infected cells) and magenta (endothelium) fluorescence; (A'') green (infected cells) fluorescence; (A''') magenta (endothelium) fluorescence. White and yellow arrowheads: infected areas close to but not part of the vasculature. (B) CHIKV infection, fixed sample labeled for GFP and RFP, from Fig. (3D'). (B') merge of green (infected cells) and magenta (endothelium) fluorescence; (B'') green (infected cells) fluorescence; (B''') magenta (endothelium) fluorescence. White and yellow arrowheads: areas of colocalization. (C'-C''') SINV infection, fixation at 2 dpi followed by clarity treatment and immunolabeling for GFP and RFP, lateral views; (C') Entire larva, boxes indicate the magnified areas in (C'') and (C'''); (C'') 3D rendering of the heavily infected area encompassing gills, liver, and hindbrain. See Movie 3, for rotation of the 3D structure; (C''') High magnification of the infected area in the tail region. White arrowheads: infected cells close to but not part of the vasculature. Scale bars: 150 μm. |
|
Infection of zebrafish larvae with other SINV and CHIKV strains. (A and B) Characterization of SINV339-mCherry strain in zebrafish WT larvae. (A) Survival curves of control uninfected (No V) and SINV- or SINV339-mCherry infected zebrafish larvae. Data pooled from 5 independent experiments. N = 60 larvae per group. (B) Expression levels of E1 SINV gene, measured via qRT-PCR, for SINV and SINV339-mCherry (2 different concentrations). Note that at the highest concentration, all SINV339-mCherry infected larvae had succumbed at 2 dpi. (C and D) Confocal image of WIHC processed SINV339-mCherry (C) and CHIKV-115 (D) infected fli1a:eGFP larvae, (C' and D') maximal projection. Scale bars: 50 μm. (C'-C'''') SINV-infected cells in magenta; vasculature in green. (C'') merge of magenta (infected cells) and green (endothelium) fluorescence from a single focal plane; (C''') magenta (infected cells) fluorescence; (C'''') green (endothelium) fluorescence. Yellow arrowheads: infected areas close to but not part of the vasculature. (D'') merge of magenta (infected cells) and green (endothelium) fluorescence from a single focal plane; (B''') magenta (infected cells) fluorescence; (B'''') green (endothelium) fluorescence. Yellow arrowheads: areas of colocalization. |