- Title
-
Cellular dynamics of regeneration reveals role of two distinct Pax7 stem cell populations in larval zebrafish muscle repair
- Authors
- Pipalia, T.G., Koth, J., Roy, S.D., Hammond, C.L., Kawakami, K., Hughes, S.M.
- Source
- Full text @ Dis. Model. Mech.
|
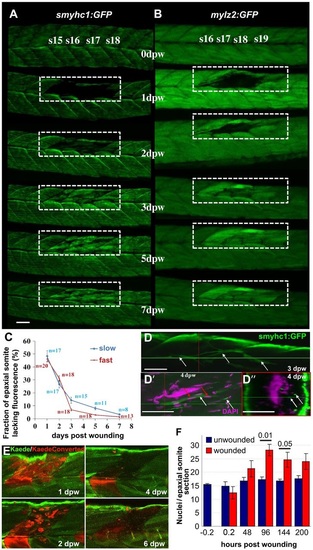
Time-course of muscle wound repair. (A,B) Large needle incision wounds (boxed regions) in the indicated somites of zebrafish 3.5dpf larvae carrying transgenes expressed in slow (A; smyhc1:gfp) or (B; mylz2:gfp) fast fibres were repeatedly imaged in the same live fish by confocal fluorescence microscopy over 7dpw. Larvae are shown anterior to left, dorsal up. Note the brighter fluorescence of newly synthesised unbleached GFP in regenerated region. s15-s19, somite 15 to somite 19. (C) Rate of recovery (mean±s.e.m.) of GFP fluorescence in epaxial somite of slow smyhc1:gfp and fast mylz2:gfp muscle of n larvae. (D-D′′) smyhc1:gfp larvae showing slow fibres (white arrows) in deep somite, viewed from dorsal (D; 3dpw) and lateral (D′) and corresponding transversal (D′′) views at 4dpw. The red and green crosshairs indicate planes, red arrows indicate elongated fibre-associated nuclei. (E) To investigate the source of new fibres, two adjacent somites in embryos injected with Kaede RNA were photoconverted from green to red at 2.5dpf, then wounded in the epaxial domain and followed for 6dpw. Representative confocal slices in lateral view show loss of KaedeRed without replacement by KaedeGreen. (F) Loss and gain of nuclei (mean±s.e.m.) in epaxial somites of Tg(h2afva:H2AFVA-GFP)kca66 larvae wounded at 3.5dpf and imaged until 12dpf (ANOVA, n=4). Scale bars: 50µm. EXPRESSION / LABELING:
|
|
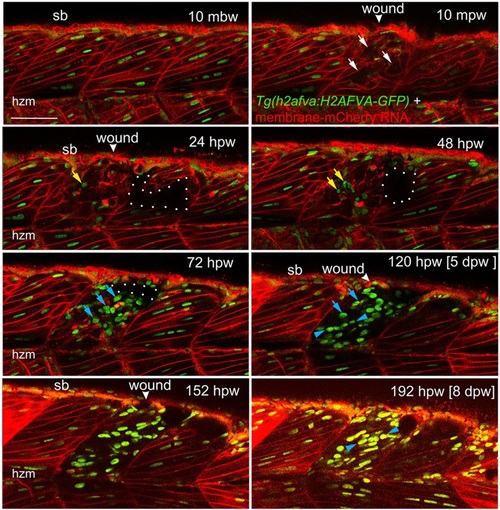
Muscle fibre regeneration in confocal time-lapse microscopy. Larvae from the Tg(h2afva:H2AFVA-GFP)kca66 line injected with membrane-mCherry RNA were wounded in epaxial somite 17 at 3.5dpf and imaged by 3D confocal time-lapse microscopy for 200hpw at 22°C. Parasagittal views are single optical slices at indicated time points from the full time series (see Movie 1). Disruption of fibres is evident immediately after wounding (white arrows). Scan shadow cast by a melanophore migrating near the wound is outlined (white dots). After loss of elongated fibre nuclei, cells with small round nuclei accumulate in wound (yellow arrows). Photobleaching resulting from scanning is evident at later times, but abundant large nuclei are located in wounds after 48hpw (blue arrows). By 5dpw, numerous rows of bright aligned nuclei are apparent (blue arrowheads). mbw, mpw, hpw and dpw: minutes before, or minutes, hours or days post-wounding; hzm, horizontal myoseptum; sb, somite border. Scale bar: 50µm. |
|
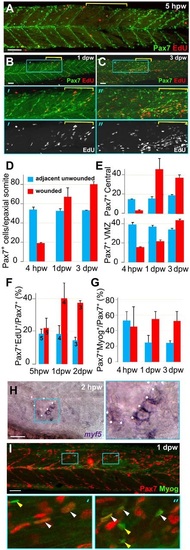
Rapid recovery of Pax7-expressing cells in wounded somites through proliferation and relocation enhances differentiation in central myotome. Wild-type zebrafish larvae wounded at 3dpf in epaxial somites 16-18 (yellow brackets) were analysed at the indicated times post-wounding by confocal immunodetection of Pax7 with EdU (A-F) or Myogenin (G,I) or in situ mRNA hybridisation for myf5 (H), shown in lateral view, anterior to left, dorsal to top. Blue boxes are magnified. (A-C) Diminished numbers of Pax7+ cells after wounding (A) are rapidly replaced (B) and show increased proliferation (B,C). (D-G). Pax7+ cells were counted in 2-4 wounded and 2-4 adjacent unwounded somite regions per larva and averaged to yield a value for each animal. VMZ, vertical myoseptum. Mean±s.e.m values from four larvae (D,E,G) or the number indicated (F). Statistical analysis is shown in Fig. S3A. (H) myf5 mRNA adjacent to a hypaxial wound (outlined by dots). Note the lack of myf5 mRNA in unwounded somites at this stage. (I) Pax7+Myog+ nuclei (white arrowheads) generally have lower Myog signal than Pax7-Myog+ cells (yellow arrowheads). Scale bars: 50µm. EXPRESSION / LABELING:
|
|
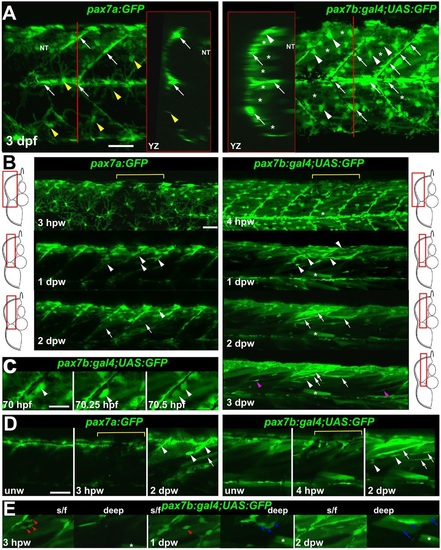
pax7a- and pax7b-reporter transgenes express distinctly during wound repair. (A) Confocal maximum intensity projection of stacks of whole somites in lateral view, showing the distribution of GFP+ cells at 3dpf in unwounded pax7a:GFP and pax7b:gal4;UAS:GFP fish. Note xanthophores (yellow arrowheads), dermomyotomal cells (white arrowheads), VMZ and HZM cells (white arrows), and labelled fibres (asterisks). Transverse YZ slices were taken at the red line. NT, neural tube. (B) Live confocal time lapse imaging of short stacks taken from the volumes indicated in the adjacent transverse schematics, shown in lateral view, anterior to left, dorsal up. Note loss of signal at 3-4hpw in regions wounded at 3dpf (yellow brackets), and recovery of GFP+ cells (arrowheads) and muscle fibres (arrows) over the ensuing days. Asterisk indicates persistence of a deep fast fibre marked by pax7b-reporter prior to wounding. Unwounded somites also accumulate small numbers of marked mononucleate cells (magenta arrowheads). (C) Time series confocal slices showing pax7b-reporter+ cell division (arrowheads) prior to wounding. (D) Magnified confocal slices showing wounding (yellow brackets) and repair. Note the stronger fibre labelling (arrows) with pax7b-reporter than with pax7a:GFP, relative to mononucleate cells (arrowheads). (E) Time series confocal slices showing superficial (s/f, left panels) and deep (right panels) pax7b-reporter+ cell appearance in the wound region followed by fusion. Disappearance of small bright GFP+ cells amongst the superficial fibres (red arrowheads) correlated with appearance of small bright GFP+ cells in deep regions (blue arrowheads, centre). Loss of some small deep cells then correlated with appearance of weakly GFP-labelled fibres (blue arrows; rightmost panel). Asterisks indicate a deep fast fibre marked by pax7b-reporter prior to wounding. Scale bars: 50µm. |
|
Fusion of pax7a- and pax7b-reporter cells during wound repair. (A-C) Lateral confocal maximum intensity projection stacks of pre-wounded (A) and wounded (B,C) yolk extension somites of pax7a:GFP;pax7b:gal4;UAS:RFP (A,B) or single pax7a/b:GFP (C) larvae, anterior to left, dorsal to top. Scale bars: 50µm. (A) At 3dpf, pax7b:RFP fibres (white arrows) and presumptive mononucleate cells (cyan arrowheads) are present superficially (s/f) within the somite and differ from pax7a:GFP cells (blue arrowheads). Dual-labelled somite cells (magenta arrowheads) concentrate on VMZ. Note the lack of Pax7 cells in the deep myotome at this stage. The pax7b-reporter labelled cells strongly in somites, and also weakly in dorsal neural tube (NT). (B) Short stack of epaxial wounded region shown by white box in A with two small wounds (asterisks). At 1dpw, pax7a:GFP;pax7b:RFP cells elongate in wound. By 2dpw, time-lapse reveals several nascent fibres marked strongly by RFP and weakly by GFP. See Fig. S9 for separate monochrome images. (C) Time-lapse of pax7b:gal4;UAS:GFP reporter marks aligned cells (arrowheads) that form fibres (top) or disappear (centre). pax7a:GFP cells are frequently aligned with fibres, but more rarely assemble in rows. pax7a:GFP cells occasionally matured into nascent fibres (bottom). Asterisks mark the same cells at each time point. Note the stronger mononucleate cells and more abundant fibre labelling by the pax7b:GFP reporter, compared with the pax7b:RFP reporter in panel B. Arrow indicates a separate cell. (D) Counts of numbers (mean±s.e.m.) of red, green and dual-labelled cells in a single epaxial somite (or corresponding length of neural tube) by cell type in larvae transgenic (Tg) for pax7a:GFP (a, green), pax7b:GFP (b, green) or pax7b:RFP (b, red) as indicated by the Tg line letter code and colour. Larvae with (+) or without (-) a wound made at 3dpf were analysed 1dpw, at 4dpf. Note the increase in labelled MPCs, decrease in fibres and constant number of xanthophores and neurons in wounded somites at 1dpw. Letter groups (m,n,p,q) indicate difference at P<0.05 (t-test, n=3). (E) At 1dpw, despite a similar fraction of total cells in myotome, there were more pax7b:GFP reporter cells in rows of two (≥2) or four (≥4) or more aligned cells, compared with pax7a:GFP cells. Mean±s.e.m., P-values show Mann-Whitney test of differences in proportions of total cells. EXPRESSION / LABELING:
|
|
Fusion of pax7a- and pax7b-reporter cells to existing myotubes during wound repair. Extended orthogonal projection views of an epaxial somite wound in a pax7a:GFP;pax7b:gal4;UAS:RFP 4dpf larva showing individual pax7a:GFP;pax7b:RFP dual-labelled (yellow) MPCs fusing to existing unlabelled (A) or RFP+ (B) fibres from Movies 2 and 3. The whole image was non-linearly enhanced and brightness corrected to compensate for bleaching and facilitate tracking of individual cells, as described in Materials and Methods. (A) At 25.5hpw, prior to fusion, an MPC had uniform cytoplasmic GFP and diffuse cytoplasmic and vesicular RFP (arrow). 10min later, cytoplasmic GFP and RFP have now filled the whole cytoplasm of a large adjacent previously unlabelled fibre (brackets), whereas the vesicular RFP remains localised (arrowhead) and integrates into the fibre in the succeeding 50min (see Movie 2, blue MPC). Transverse II shows the same view as Transvere I, but with the fusing fibre marked (dots). (B) At 39.5hpw, two MPCs (magenta and yellow arrows), fuse simultaneously to the same large adjacent myotube (arrowheads; Movie 2, magenta and yellow MPCs). (C) At 32.3hpw, a dual-labelled MPC (arrowhead; Movie 3, white MPC) that originated from the anterior somite border, divided and then migrated along a recently formed GFP+ nascent myofibre (arrow). 10min later the MPC has fused into the nascent fibre, as shown by RFP loss from the MPC and increase in the fibre. The fused cell remains distinct at 10min, but merges into the nascent fibre by 20min. Process shown in merge and single colour lateral (dorsal up, anterior left) and transverse (dorsal up, medial left) views. Blue lines indicate range of extended orthogonal projection views. |
|
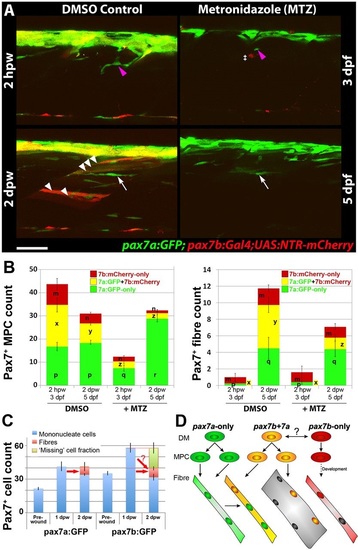
Ablation of pax7b-expressing cells diminishes marked fibres. pax7a:GFP;pax7b:NTR-mCherry larvae were treated overnight with MTZ or DMSO vehicle, then wounded and imaged by confocal 3D scanning at the indicated times. (A) Representative time-lapse images of wounded epaxial somites, showing MPC (magenta arrowheads) invasion of wound at 2hpw, followed by formation of nascent fibres at 2dpw (white arrows). Numerous large dual-labelled fibres are observed in control (white arrowheads). Anterior to left, dorsal to top. Scale bar: 50µm. (B) Counts of numbers (mean±s.e.m.) of red, green and dual-labelled MPCs and fibres in single wounded epaxial somites of control and MTZ-treated larvae. Counts omitted fast-moving phagocyte (‡ in A) and transient fibre labelling, which appeared rapidly only after wounding in MTZ-treated NTR-mCherry larvae and reflect the abundant release of mCherry from dying cells and uptake into injured fibres or phagocytes. Counts of each labelled cell type were compared between DMSO- and MTZ-treated larvae at 2hpw and 2dpw by two way ANOVA (with Bonferroni post hoc test, n=4 DMSO and 5 MTZ; for complete dataset and statistical analyses see Table S1). Within each cell type, distinct letters on columns (m,n,p,q,r,x,y,z) show groups that differed statistically at P<0.05. (C) Quantification (mean±s.e.m.) of mononucleate cells in epaxial wounded somites of pax7a:GFP or pax7b:GFP larvae reveals an increase at 1dpw, followed by a decrease at 2dpw, accompanied by formation of labelled fibres. Note the similar number of labelled fibres in each line (red arrows), and the apparent substantial reduction of labelled cells (‘missing’ fraction) only in the pax7b:GFP reporter line. (D) Modified founder cell model illustrating the known and hypothesised (?) behaviour of the pax7a:GFP-only MPC population (green), which contribute to wound repair and form nascent fibres, and the pax7b MPC population (yellow), which is more abundant, fuses to pre-existing (damaged?) fibres in the region of the wound and contributes to nascent fibre growth. We hypothesise that pax7a-only cells are founders initiating new fibre formation, whereas pax7b cells are FCMs contributing to fibre growth both in wounding and normal development. pax7b:RFP-only MPCs (red) form fibres early in development, accumulate at HZM and might act as a stem cell. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Cellular changes in wounded muscle. A. Lateral confocal image stack projections of wounded epaxial somites (red arrowhead) in larvae wounded at3 dpf and analysed 4 hours post wounding (hpw) or 1-5 dpw. Fixed larvae were stained for F-actin (Phalloidin) and nuclei (Hoechst). Note the broken fibres (asterisks) and rounded nuclei at the 4 hpw wound site. Loss of regular striation and presence of actin aggregates at 1 dpw (arrows) is followed by accumulation of nuclei in the wound site at 2 dpw (arrowheads). At 2 dpw, striations begin to reappear in thin fibres (yellow arrowheads) at the wound edge, and are abundant by 4 dpw. Dorsal to top, onterior to lcft. Bar = 50 µm. B. Quantification of nuclei per confocal transverse cross-section of epaxial somites that were wounded (red), adjacent to wound but unwounded (purple) and in unwounded fish (blue). Half the nuclei at the horizontal myoseptum were attributed to the epaxial somite. |
|
Epidermal and leukocyte movements during wound closure. A-C. Rapid epidermal wound closure in a 2.5 dpf membrane-mCherry RNA-injected Tg(h2afva:H2AFVA-GFP) larva in optical parasagittal section (left), taken at the level of the white dotted line in the transverse sections (right). A. Before wounding, polygonal epidermal cells (ep) overlie superficial slow muscle cells (ssm). B. At 10 mpw, an epidermal ring already formed (white arrow) and was almost closed at 60 mpw (C, white circle and arrow). Transverse sections show that the epidermal region above the wound thickens oy some 10-15 µm. D. Tg(lyz:EGFP) fish were wounded around s12 at 2.5 dpf (red arrows) and imaged in lateral view with dorsal up and anterior to left. GFP+ cells accumulated at wound site 4 hpw. E. Tg(mpx:GFP) and Tg(lyz:EGFP) were wounded in two to four somites between epaxial s5-12 at 2.5 dpf. GFP+ cells were counted at the wound site of the number of fish indicated. F. Change in numher of hright mCherry cells (arrowheads) with time in membrane-mCherry RNA-injected Tg(h2afva:H2AFVA-GFP) larva wounded at 2.5 dpf. a - anterior, d - dorsal. 1- lateral. m - medial, nc - notochord, nt - neural tube, p - posterior. v - ventral. Bars = 50 µm. |
|
Most nuclei in wound sites have undergone S-phase. Tg(Ola.Actb:Hsa.HRAS-EGFP) larvae were wounded at 3 dpf in epaxial somite 517 and treated with EdU (left) or control vehicle (right) from 4 hpw until 3 dpw/6 dpf, followed by immunodetection of anli-GFP-Alexa488, ClickIT-555 for EdU and Hoechst for DNA. 3D confocal stacks were processed with Imaris to make short orthogonal projections from the planes indicated oy the blue markings. The wounded somite region (white bracket) contains abundant nuclei, most of which are EdU-marked. Many nuclei have the typical elongated form and alignment of fibre nudei (arrows). Other regions of the wound still contain nuclei with rounded morphology (asterisks). The transgene membrane GFP labels some regenerating cells weakly (right), for reasons that are unclear. Adjacent unwounded somites contain numerous nuclei unmarked by EdU (arrowheads) and some EdU-marked nuclei, possibly reflecting normal growth. Note the abundant EdU label in superficial cells reminiscent of dermomyotome (yellow arrowhead). 16/20 EdU-treated individuals showed all these features. Bars = 20 µm. |
|
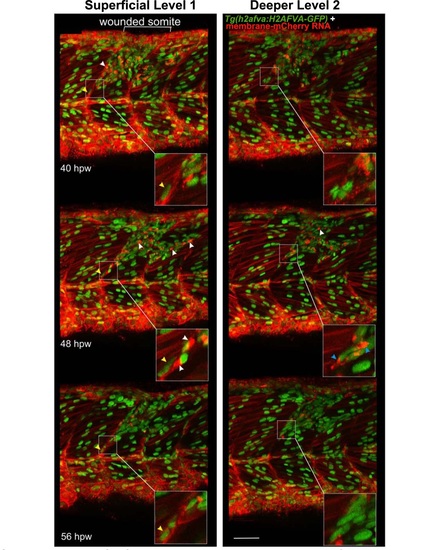
Cells with specialised morphology at borders of wounded somites. Time-lapse of short stacks of lateral confocal sections from larva in Movie 81 , anterior to left. Wounded region is indicated (white bracket). Level 1 is about 30 µm more superficial than Level 2 revealing the extent of damage. Boxed insets show vertical myoseptal (VMZ) region. Some cells aligned with the VMZ (yellow arrowheads) move little between time-points. Other cells aligned at the VMZ had a small oval nucleus and a bright ′cap′ of membrane mCherry (white arrowheads) and appeared at the lesion site between 32-40 hpw, but could not be identified at the VMZ at subsequent time-points, suggesting rapid migration or change of appearance. At 48 hpw, such ′capped′ cells were dispersed in the central myotome and more numerous, but their number subsequently declined. Some cells at the VMZ appeared to have unusually elongated nuclei and bright bipolar cytoplasmic mCherry, suggesting that they might be dividing cells (blue arrowheads). The labelling of all cells and the low time resolution of the movie prevented observation of such events as MPC proliferation and fusion in real time. Bar = 50 µm. |
|
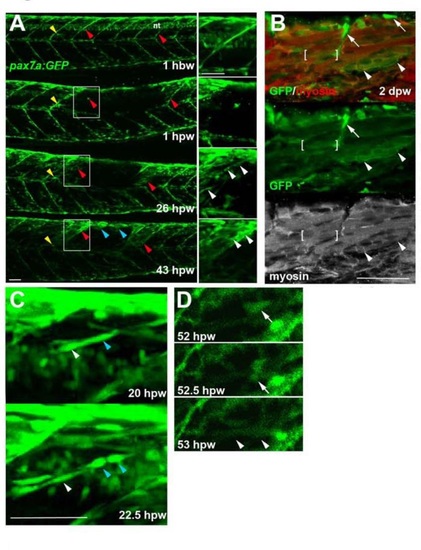
Genetically marked pax7aexpressing cells contribute to regeneration. Pax7a:GFP zebrafish larvae wounded at 3-4 dpf were repeatedly confocally scanned live embedded In agarose (A,e,D) or fixed and stained (8). All larvae are shown in lateral view, anterior to left, dorsal up. Repair of large wounds followed a variable spatiotemporal course, likely reflecting variability in the extent of initial wounding. Whereas in some cases repair seemed faster near to unwounded somites (A), in other cases recovery at the dorsal edge of wounded somites was more rapid and repair progressed in parallel in all somites (see Fig. S7). A. Time lapse of pax7a:GFP;pfe/pfe larva wounded at 4 dpf in the epaxial reg ion of somites 16-20. Prior to wounding (top panel), GFP is present in neural tube (nt), somites borders and a residual xanthophore (yellow arrowheads), which marks the anteriormost wounded somite. Note the recovery of GFP in central myotome of somites that retained GFP+ cells at vertical myosepta (red arrowheads) and spreading from dorsal epaxial edge (blue arrow- heads). Boxed regions, magnified at righl. show elongating GFP+ cells in central somite 16 at 1 dpw (white arrowheads) yielding thin muscle fibers by 2 dpw. In large wounds, most pax7a:GFP signal was lost at the wound site, consistent with ablation of many muscle precursor cells. By 1 dpw, pax7a:GFP+ cells began to re-accumulate from the edge of the wounded region, occupying the centre of wounded somites and seeming to elongate (insets). AI 2 dpw. pax7a:GFP-expressing cells contributed to muscle fibre regeneration, as demonstrated by the appearance of GFP in elongated multinucleate muscle fibres containing myosin heavy chain (compare A with B). B. Immunodeteclion of pax7a:GFP and myosin heavy chain (MyHC) two days after wounding two adjacent somites revealed mononucleate GFPstrongMyHC cells (arrows), thin GFPmediumMyHCweak nascent fibres with unstructured MyHC (arrowheads) and larger GFPweakMyHC+ maturing fibres with recognisable sarcomeric structure (between brackets). The pax7a:GFP signal rapidly weakened as fibres enlarged, often requiring immunodetection to reveal the GFP in maturing regenerated fibres. Strikingly though, the number of pax7a:GFP-labelled cells in somites in the central region of large wounds remained low; most labelled mononucleate cells were present in somites at the wound edge, where new GFP-labelled fibres also first appeared (see A 43 hpw). It therefore appears that pax7a:GFP marked cells at wound edges initially contributed to muscle regeneration. C. Brief time lapse series of short stacks showing pax7a:GFP+ cells dividing (blue arrowhead) and migrating (white arrowhead). Such detailed time-lapse analysis confirmed that pax7a:GFP marked cells migrate and divide, consistent with their re-accumulation in the lesion. D. Brief time-lapse series of single plane showing pax7a:GFP+ cell moving (arrows) and then fusing into a fibre (arrowheads). After entering the wound a proportion of cells fuse into nascent fibers. These results show that Pax7a+ cells at the dorsal and vertical somite border can initiate fibre regeneration. Bars = 50 µm. |
|
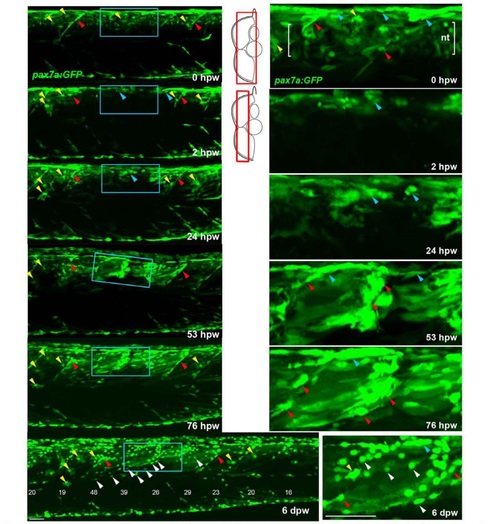
Genetically marked pax7a-expressing cells contribute to regeneration. Tg(pax7a:GFP):pfe/pfe zebrafish larva was wounded at 4 dpf in the epaxial region of somites 16-19 and repeatedly confocally scanned live, embedded in agarose. Maximum intensity projections of selected regions of the stack are shown in lateral view, anterior to left, dorsal up. Only top panel includes neural tube levels (red boxes in schematics). Blue boxed regions, magnified at right, show GFP+ cells near dorsal epaxial edge in central somite 16 at 1 dpw yielding thin muscle fibres by 2 dpw (blue arrowheads). Prior to wounding (top panels), GFP is present in cells of neural tube (nt. between brackets), dorsal and vertical somite borders (blue and red arrowheads, respectively) and rare xanthophores (yellow arrowhead). In contrast to the larva in panel S6A, GFP recovers more rapidly in central wounded somites (blue box) that had GFP+ cells spreading from dorsal epaxial edge at 24 hpw (blue arrowheads). pax7a:GFP+ cells began to re-accumulate at the dorsal edge by 1 dpw and fibre formation was well underway by 2 dpw. As nascent fibres matured and increased in volume, their GFP fluorescent intensity declined, while small myogenic cel ls remained strongly labelled. In contrast to the larva in panel S6A, GFP recovers more rap idly in central wounded somites (blue box) that had GFP+ cells spreading from dorsal epaxial edge at 24 hpw (blue arrowheads). At 6 dpw, when wound repair was advanced, numerous small round GFP+ cells (white arrowheads) appear in all somites overlying the myotome, in the dermomyotome location. These cells were more numerous in wounded somites than in adjacent unwounded somites, and concentrated at the posterior somite border. In summary, pax7a-expressing myogenic cells contribute to muscle repair and a sub-population of such cells remains undifferentiated after myotome recovery. Bar = 50 µm . |
|
Individual green and red channels showing fusion of pax7a- and pax7b-reporter cells during wound repair (from Fig. 6A,B). Lateral confocal maximum intensity projection stacks of pre-wound (upper three rows. from Fig. 5A) and post-wound (lower two rows. from Fig. 58) yolk extension somites of a pax7a:GFP;pax7b:RFP larva, anterior to left, dorsal to top. A. At 3 dpf, prior to wounding, xanthophores have strong GFP and weak RFP, neural tube cells have strong GFP and little RFP, whereas superficial muscle fibres have RFP but lack GFP. B. Short stack of epaxial wounded somite region excluding deep and superficial regions. Prior to wounding, no pax7a:GFP cells and only a pax7b:RFP large fibre are present in the deep somite. Two oblique needle insertions made two small lesions in a single epaxial somite (white arrows). By 1 dpw, rare pax7a:GFP-only cells (blue arrowheads) and abundant dual·labelled cells (magenta arrowheads) elongate in wound. Rare weak pax7b:RFP cells with little or no GFP (cyan arrowheads) are also present. By 2 dpw, time-lapse reveals several nascent fibres marked strongly by RFP and weakly by GFP. Rounded pax7b:RFP cells are still present in the wound reg ions, but pax7a:GFP-only cells are diminished. Bars = 50 µm |
|
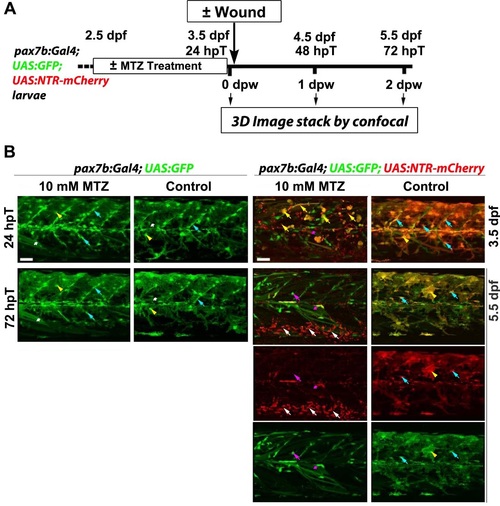
MTZ specifically ablates pax7b:NTR-mCherry cells. A. Work flow of metronidazole (MTZ) mediated selective ablation of cells expressing nitroreductase (NTR) from pax7b-driven NTR-mCherry cells (hpT. hours post start of MTZ treatment; dpw. days post wound; dpf. days post fertilisation). B. Larve of the indicated genotype were treated with 10 mM MTZ or DMSO vehicle/control from 2.5 dpf to 3.5 dpf and not wounded. Lateral maximum intensity projection of stacks from the same (except righthand column) 24 hpT/3.5 dpf and 72 hpT/5.5 dpf embryos are shown. Bottom two rows show single channels of 5.5 dpf merge above. Controls lacking NTR (two lefthand columns) show that MTZ is not toxic without NTR. Marked fibres (white asterisks), xanthophores (yellow arrowheads) and MPCs (cyan arrowheads) survive treatment. In control DMSO-treated dual-labelled fish. GFP and mCherry largely overlap at 3.5 dpf in MPCs and xanthophores (right column). MTZ treatment of NTR-expressing larvae eliminated most of the mCherry MPCs and xanthophores. leaving dying cells (yellow arrows) that were eliminated by 72 hpT. Low numbers of GFP-only cells and fibres remained (magenta arrow and asterisks). By 3 days after MTZ treatment. almost all mCherry debris had been cleared and numerous motile phagocytes were present ventrally around major blood vessels (white arrows; GFP had been lost immediately upon engulfment). GFP shows reduction of pax7b-expressing cells. No re-appearance of mCherry was observed in MPCs or other cells. Bars = 50 µm. |