- Title
-
Optical micromanipulation of nanoparticles and cells inside living zebrafish
- Authors
- Johansen, P.L., Fenaroli, F., Evensen, L., Griffiths, G., Koster, G.
- Source
- Full text @ Nat. Commun.
|
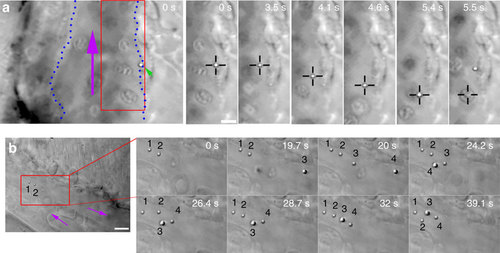
In vivo optical micromanipulation of microinjected particles. (a) A particle (green arrowhead) adhered to the endothelium of the caudal vein (indicated with blue dotted lines) is pulled away from the endothelium into the fast blood flow (purple arrow) using optical tweezers (black crosshairs). At time 5.5 s an erythrocyte is drawn into the trap. This replaces the particle in the trap which is subsequently pulled back towards the original adhesion point of the endothelium, presumably due to a connecting nanotube. Experiment repeated at least 80 times. (b) Four separate particles (numbered) are fished out of the blood flow and moved towards a sheltered region at the tip of the tail. Purple arrows indicate flow direction. Experiment was repeated at least 10 times. Scale bar, 5 µm. |
|
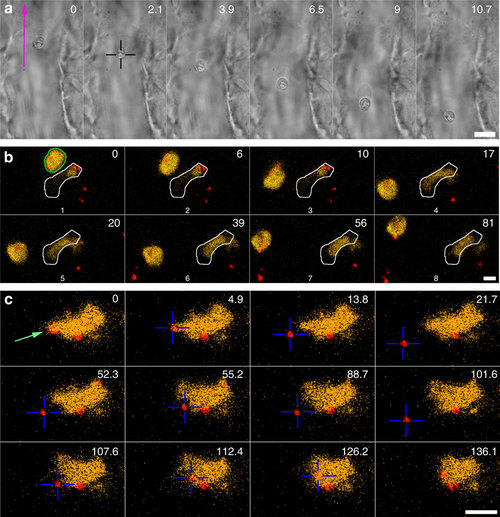
rapping of erythrocytes and macrophages. (a) An erythrocyte is trapped and moved in the blood flow. Scale bar, 10 µm. Experiment repeated at least 10 times. (b) A blood-resident fluorescent macrophage (yellow, green outline in t=0 s) was micromanipulated and moved in 3D in a blood vessel. The white outline indicates another, non-mobile macrophage. The red dots are injected particles. Scale bar, 5 µm. Experiment repeated at least 5 times. (c) An injected particle (red colour) that was associated with a macrophage was tested for adhesion. First the particle was moved away (t=0-21.7 s) after which the OT was briefly shut off. This did not result in the particle flowing away with the blood, suggesting that a nanotube (not visible) was tethering the particle; next the particle was carefully brought into contact and moved away again (52.3-101.6 s), indicating that no strong binding was established. Finally, the particle was moved further into the macrophage with a higher pushing force after which the particle could not be detached anymore (t=107.6-136.1 s). Scale bar, 10 µm. Experiment was repeated at least 5 times. |
|
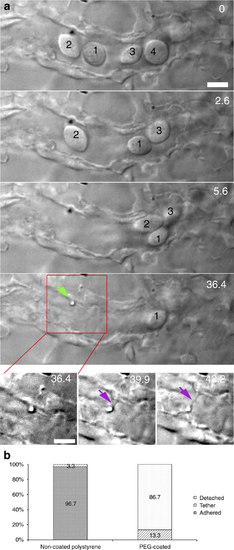
Multiple traps for in vivo nanotube formation in cell-free blood vessels. (a) In a smaller blood vessel several erythrocytes are cleared (0-36.4 s) and fenced off, after which an adhered particle was moved away from the endothelium, and a tethering nanotube was formed (36.4-42.2 s). Scale bar, 5 µm. (b) Quantification of the adhesion probability of naked and PEG-coated particles, classified as: detachable (white), strongly adhered (solid) and tethered (lines). Experiment was repeated at least 60 times. |
|
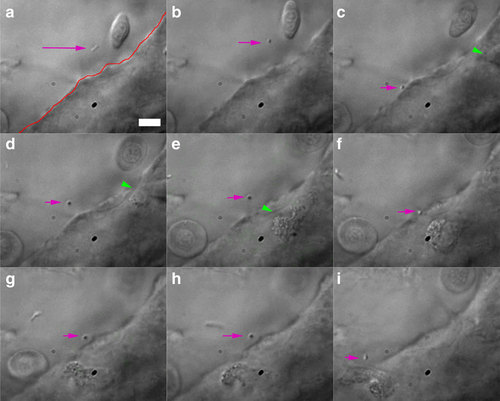
Trapping of injected bacterium. (a) A diffusing bacterium (purple arrow) is (b-d) trapped, pushed against and moved away from the endothelium (red line in a). This seems to activate an immune cell (green arrowheads), which moves towards the contact point (c-f). Repeated contact with the endothelium seems to again attract the attention of the crawling cell, which (g-i) finally moves into the vein. The total duration of experiment is ~2 min. Scale bar, 10 µm. Experiment was repeated at least 4 times. |
|
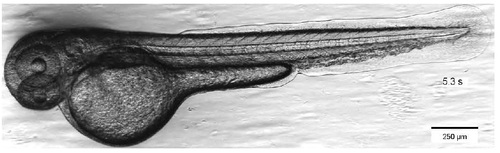
Transmission bright field image of zebrafish larva. Snapshot of Supplementary Movie 1. |
|
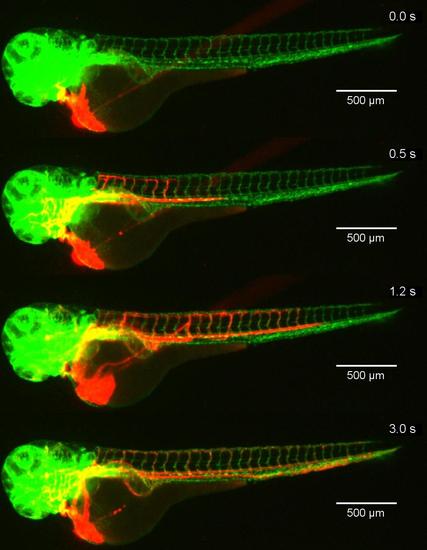
Injection of nanoparticles into the zebrafish with blood vessels with green fluorescent protein labeled endothelium. Snapshots of Supplementary Movie 2. At t=0 microinjection was started. The particles (fluorescent, red) distribute throughout the zebrafish within 3 seconds. |
|
Two particles are trapped from the blood in the tail region of the fish and moved towards the endothelium simultaneously. Snapshots of Supplementary Movie 5. Experiment repeated at least 10 times. Time in seconds. Bar is 10 µm |
|
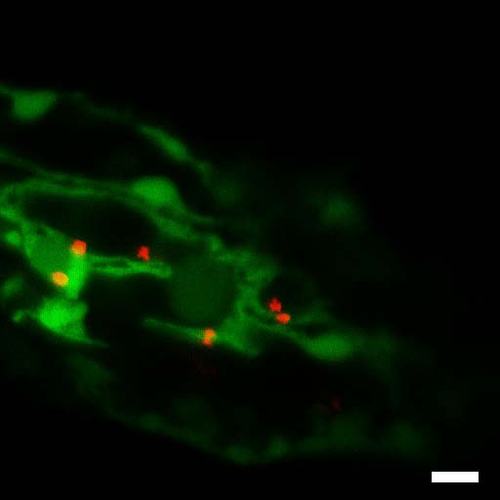
Trapping of fluorescent (red) particle in zebrafish with fluorescent (green) endothelium. Snapshot of Supplementary Movie 8. Experiment repeated at least 10 times. Bar 10 µm. |