- Title
-
sept7b is essential for pronephric function and development of left-right asymmetry in zebrafish embryogenesis
- Authors
- Dash, S.N., Lehtonen, E., Wasik, A.A., Schepis, A., Paavola, J., Panula, P., Nelson, W.J., and Lehtonen, S.
- Source
- Full text @ J. Cell Sci.
|
Septin 7 is expressed throughout zebrafish development and is enriched in the brain and pronephros. sept7b mRNA expression in zebrafish larvae (A–E). (A) By using qRT-PCR and normalizing to rps3 (ribosomal protein subunit 3), it can be seen that sept7b is expressed in fertilized embryos at 0 hpf, 2 hpf and in 1–4-dpf embryos and larvae. (B) In situ hybridization shows that sept7b is expressed in 3-hpf embryos. (C–F) Lateral views of 4-dpf larvae hybridized with sept7b antisense (C,E) and sense (D,F) probes. (C,E) sept7b mRNA is concentrated in the brain and the pronephric tubule (pt). (D,F) The sense probe shows no signal, except in the swim bladder region (D). (G) A section of a 2-dpf embryo shows sept7b mRNA in the glomerulus (arrow labeled g) and the pronephric tubule (arrowheads). Notochord, nc. (H–K) The septin 7 protein is localized in the pronephric tubule and glomerulus. (H,I) Immunostaining shows that septin 7 (red) localizes in the pronephric tubule cells (t; arrowhead), and in the glomerulus (g) of 4-dpf wt1b::GFP zebrafish larvae (J,K). (L–S) Septin 7 is expressed in ciliated cells in 72-hpf embryos. Cilia are visualized by staining for acetylated tubulin (red). Septin is indicated by green fluorescence. (L) Septin 7 localizes in pronephric tubule cells. (M) In addition to localizing in the cytoplasm of pronephric tubule cells, septin 7 concentrates at the base of or along the cilia (arrows). (N) Septin 7 is found at the base of, or along, the cilia in the central canal (arrows). (O) Septin 7 is expressed in nasal epithelial cells, along the ciliary axoneme and in the tips of cilia. (P) Septin 7 is localized along the axoneme of the neuromast in the head region (arrows). (Q) In lateral line neuromasts, septin 7 is found close to the base of cilia (arrows). (R) In the cloaca region, septin 7 is found in the epithelium and at the base of cilia (arrows). (S) Higher magnification reveals a concentration of septin 7 at the base of cilia in cloaca (arrow). Scale bars: 100μm (B,E,F); 300μm (C,D); 20μm (G,R); 50μm (H,I); 40μm (J,K); 10μm (L–O); 7.5μm (P); 2.5μm (Q); 3μm (S). |
|
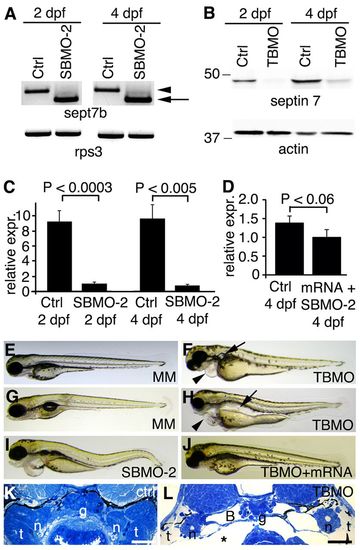
sept7b knockdown causes body curvature, edema and pronephric cysts. (A) PCR confirms skipping of exon 2 (arrow; amplicon 626bp) of sept7b in SBMO-2-injected embryos at 2 and 4 dpf. Arrowhead marks the non-spliced sept7b amplicon (726bp). Rps3 is used as a control (amplicon 428bp). Ctrl, control. (B) Immunoblotting of 2- and 4-dpf larvae that had been injected with TBMO indicates that TBMO efficiently blocked translation of sept7b mRNA. Actin is used as a loading control. (C) qRT-PCR indicates that the expression level of sept7b is reduced by 89–92% in SBMO-2-injected embryos at 2 and 4 dpf. Rps3 was used to normalize expression values. (D) qRT-PCR of 4-dpf larvae shows that the expression level of sept7b is partially restored in rescue experiments when capped zebrafish sept7b mRNA is co-injected with the sept7b SBMO-2. (E,F) Injection of the sept7b TBMO into embryos causes pericardial (arrowhead) and yolk sac edema, and pronephric cysts (arrow) at 3 dpf (F), whereas the TBMO-MM-injected embryos (MM) do not show phenotypic differences (E). (G,H) sept7b TBMO-injected larvae (H) show pericardial (arrowhead) and yolk sac edema, body axis curvature, and dilation of pronephric tubules (arrow) compared to the TBMO-MM-injected larvae at 4 dpf. (I) sept7b SBMO-2 injection causes pericardial edema and body axis curvature. (J) The phenotypic differences can be rescued by co-injection of sept7b capped mRNA with the sept7b TBMO. (K) Histological section of a 4-dpf control larvae. (L) sept7b morphant larvae at 4 dpf shows a dilated Bowman′s space (B). g, glomerulus; n, neck segment of the pronephric tubule; t, pronephric tubule; asterisk, edema. Scale bars: 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
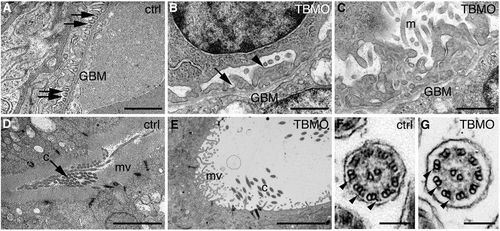
Depletion of sept7b causes defects in the structure of the pronephros. (A–E) Electron micrographs of control and sept7b-morphant kidneys at 4 dpf. (A) Wild-type podocytes show regular foot processes connected by slit diaphragms (arrows). Glomerular basement membrane, GBM. (B,C) Podocytes of a sept7b morphant (TBMO) show foot process effacement (arrowhead) and microvillar-type cell processes (m) on their apical surface. Occasional slit diaphragms (arrow) were observed. (D) A pronephric tubule of the wild-type larva (ctrl) shows regular microvilli (mv) and cilia (c) morphology. (E) A tubule in a sept7b morphant appears dilated and shows sparse and irregular microvilli and scattered cilia. (F,G) The cilia in both control (F) and TBMO morphants (G) show the normal 9+2 structure of microtubules. The outer dynein arms are visible in both the control and sept7b morphant cilia (arrowheads). Scale bars: 1μm (A–C); 10μm (D,E); 140nm (F,G). PHENOTYPE:
|
|
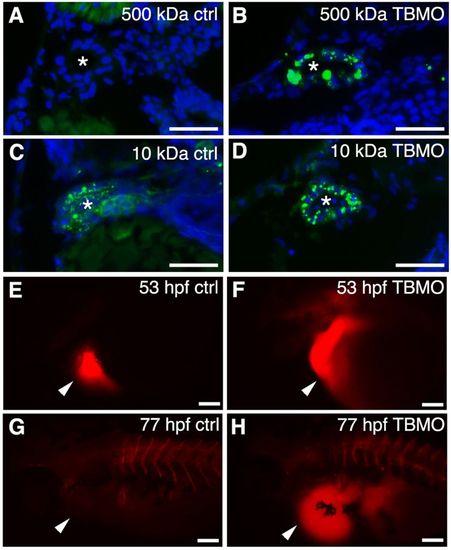
Knockdown of sept7b affects glomerular barrier function and fluid flow. (A,B) 500-kDa fluorescein dextran (green) injected into the cardinal vein of 3.5-dpf larvae passes the glomerular barrier and is endocytosed into the pronephric tubules of sept7b-TBMO-injected larvae (B), whereas in the control (ctrl; A) the dextran remains in circulation. (C,D) 10-kDa fluorescein dextran readily passes the glomerular barrier in both control (C) and TBMO-injected (D) larvae and is endocytosed into the pronephric tubules. DAPI (blue) stains the nuclei, and the asterisk marks the lumen of the tubule. (E–H) Tetramethylrhodamine dextran (70,000-kDa) was injected into the pericardial sac (arrowheads) of control (E) or sept7b-TBMO-injected (F) embryos at 53 hpf that were imaged immediately after dye injection (E,F) and again at 77 hpf (G,H). Clearance of the dye was observed in the control larvae (G) but not in sept7b morphants (H). Scale bars: 20μm (A–D); 200μm (E–H). PHENOTYPE:
|
|
Depletion of sept7b reduces the length of cilia in the pronephric tubule and Kupffer′s vesicle, and causes misorientation of basal bodies and cilia. (A) Acetylated tubulin (red) stains cilia in 30-hpf wt1b::GFP embryos. (B) Staining of 30-hpf sept7b-TBMO morphants for acetylated tubulin (red) reveals shortened cilia, indicating that sept7b is essential for ciliogenesis. (C) The length of pronephric cilia is reduced in sept7b morphants (TBMO). Ctrl, control. (D,E) Kupffer′s vesicle of control (D) and sept7b morphant (E) embryos at 12–13 hpf that have been stained for acetylated tubulin to visualize cilia. (F) The length of cilia of Kupffer′s vesicle is reduced in sept7b morphants compared with controls. (G) Immunostaining of cilia for acetylated α-tubulin (red) and of basal bodies for γ-tubulin (green) in the multi-ciliated region of the pronephric tubule at 30 hpf in control embryos shows well-organized basal bodies that localized at the base of cilia in the tubular epithelial cells. (H) In sept7b-TBMO-injected embryos, the basal bodies are disorganized (arrowheads), the tubule diameter is increased (asterisk) and cilia appear misoriented. (I) Electron microscopy reveals apically docked basal bodies with outgrowths of ciliary axonemes in 4-dpf control larvae. (J) In sept7b-TBMO-injected larvae, the basal bodies docked at the apical cell membrane but appeared disorganized (indicated by the lines showing the orientation of the basal bodies). (K,L) The ciliary angle in control (20.9°; K) and sept7b-TBMO-injected larvae (41.1°; L) differed substantially, indicating that cilia are misoriented in sept7b-depleted larvae. The lines are drawn through the central pairs of the microtubules to indicate the orientation of the cilia. Scale bars: 20μm (A,B,D,E,G,H); 1μm (I–L). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Apical actin is reduced in the pronephric tubule epithelia of sept7b-knockdown larvae. (A,B) Staining with phalloidin shows prominent apical actin (arrows) in the pronephric tubule of larvae injected with the TBMO-MM control (ctrl; A). By contrast, the intensity of actin staining is reduced in larvae injected with the sept7b TBMO (TBMO, B). The arrowheads point to muscle with intense labeling for actin. DAPI (blue) stains the nuclei. (C) The fluorescence intensity of actin staining in the pronephric tubules is reduced in sept7b morphants. The same settings were used during imaging of control and morphant larvae, and the images were analyzed without processing. Scale bars: 10 μm. PHENOTYPE:
|
|
Suppression of sept7b leads to defects in left–right asymmetry and hydrocephaly. (A–C) In situ hybridization of spaw in control (ctrl) embryos at the 20-somite stage (A) and in embryos treated with the sept7b TBMO (B,C). Spaw is expressed in the left lateral plate mesoderm in control embryos (A), whereas spaw expression is bilateral (B) or in the right lateral plate (C) in sept7b morphants, as indicated by arrows. (D–F) In situ hybridization of cmlc2 in a control embryo at the 30-somite stage (D) and in sept7b TBMO morphants (E,F). Cmlc2 expression shows the heart tube looping to the left in control embryos (D), whereas cmlc2 expression is in the center (E) or looping to the right (F) in sept7b morphants, as indicated by arrows. (G) The number of control and sept7b morphant embryos with spaw expression on the left side, right side, bilaterally or absent. (H) The number of control and sept7b-morphant embryos with cmcl2 expression on the left side, in the center or on the right side. (I,J) 2-dpf control embryos (I). (J) sept7b morphant (TBMO) at 2 dpf shows hydrocephaly (arrow). (K,L) Histological sections stained with hematoxylin–eosin of 4-dpf control (K) and TBMO-injected (L) zebrafish larvae show hydrocephalus in the morphants (arrow). (M,N) Immunofluorescence staining for acetylated tubulin (red) reveals misorientation, shortening and a reduced number of cilia in 30-hpf zebrafish that had been injected with the TBMO (N) compared with the control (M). Scale bars: 130μm (A–F); 100μm (K,L); 10μm (M,N). EXPRESSION / LABELING:
PHENOTYPE:
|
|
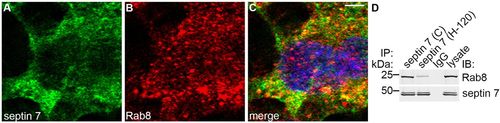
Septin 7 forms a complex with Rab8. (A–C) Immunofluorescence staining of mIMCD3 cells shows partial colocalization of septin 7 (A) and Rab8 (B) in the perinuclear region, as shown by the yellow coloring in the merged image (C). Scale bar: 10μm. (D) Two different septin 7 antibodies (C or H-120) co-immunoprecipitate Rab8 in mIMCD3 cell lysates, indicating that septin 7 and Rab8 physically interact. IP, immunoprecipitation; IB, immunoblotting. |
|
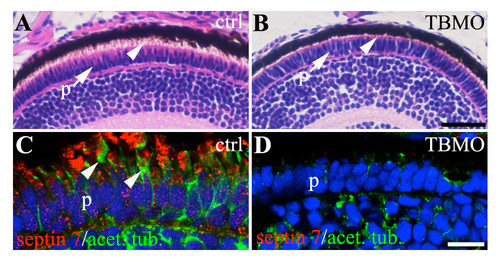
Sept7b is required for the development of photoreceptor outer segment. (A) Hematoxylin-eosin stained histological section of 4 dpf zebrafish larva showing the photoreceptor cell layer (p). The photoreceptor outer segment, that comprises modified cilia, is marked with an arrowhead. (B) Histological section of 4 dpf sept7b translation blocking antisense morpholino oligonucleotide (TBMO)-treated zebrafish larva shows shortening of the photoreceptor (p) outer segment cilia (arrowhead). (C-D) Immunofluorescence staining of 4 dpf control (C) and sept7b TBMO-treated zebrafish (D) for septin (red) and acetylated tububulin (green). (C) Septin 7 localizes in a punctate fashion in the photoreceptor cells in the control, and the photoreceptor outer segment is visualized by acetylated tubulin (arrowheads). (D) The photoreceptor outer segment cilia are absent or clearly shorter in sept7b TBMO-treated zebrafish larva. Scale bar: 25 μm (A-B); 10μm (C-D). |
|
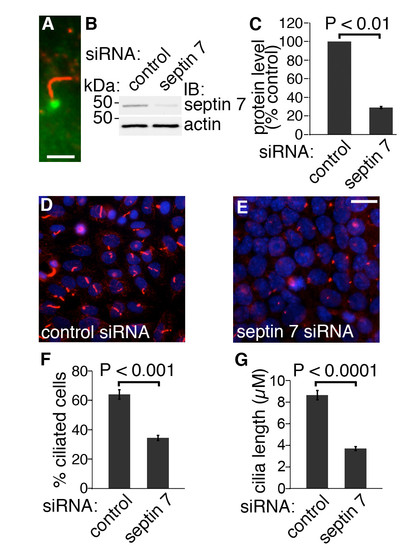
Knockdown of septin 7 decreases the length of cilia in mIMCD3 cells. (A) In mIMCD3 cells, septin 7 (green) is expressed at the base of cilia, which are stained for acetylated tubulin (red). Scale bar: 2,5 μm. (B) mIMCD3 cells were transfected with 100 nmol ON-TARGET plus SMARTpool mouse Sept7 (L-042160-01-0005) or siCONTROL Non-Targeting Pool#2 (D-001206- 14-05) siRNAs (Dharmacon, Lafayette, CO) using Lipofectamine 2000 (Invitrogen). Septin 7 siRNA leads to a 71% reduction in septin 7 expression in mIMCD3 cells. Actin was used as a loading control. (C) Quantification of protein levels of three replicate blots as in (B). (D-E) mIMCD3 cells treated with control siRNA (D) are decorated with cilia identified by staining for acetylated tubulin (red). In mIMCD3 cells transfected with septin 7 siRNA (E), the number and length of cilia are reduced. Nuclei are visualized with Hoechst stain (blue). Scale bar: 10 μm. (F) Quantification of 4000 cells indicates that 64% of cells transfected with the control siRNA had cilia whereas only 34.5% of cells transfected with septin 7 siRNA were ciliated. (G) Cilia length is reduced in septin 7 siRNA treated cells (3.69 ± 0.47 μm) compared to control siRNA transfected cells (8.86 ± 0.63 μm; n=200 for both). Graphs in C, F and G show the mean and error bars (STDEV) of three independent experiments, Student’s t-test. |