- Title
-
Loss of col8a1a function during zebrafish embryogenesis results in congenital vertebral malformations
- Authors
- Gray, R.S., Wilm, T.P., Smith, J., Bagnat, M., Dale, R.M., Topczewski, J., Johnson, S.L., and Solnica-Krezel, L.
- Source
- Full text @ Dev. Biol.
|
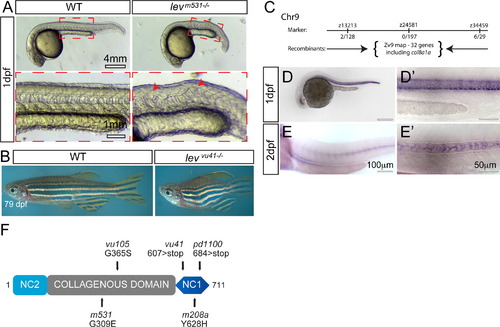
lev zebrafish mutant alleles disrupt the col8a1a gene. (A) Representative col8a1a (e.g. levm531-/-) mutant embryo displaying kinking of the notochord (red arrowhead) and shortened body axis at 1 dpf. (B) Representative homozygous col8a1a mutant adult (e.g. levvu41-/-) displays shortened body axis. (C) The levvu41 lesion was meiotically mapped to a region of chromosome 9 between the markers z13213 and z34459 (number of recombinants are noted at each region) and was most highly associated with the SNP, rs40743937 in our bulk segregant analysis. According to the ensemble map (ZV9), 32 genes are located within this critical region flanked by the SSLP markers, including the col8a1a. (D–E′) col8a1a in situ hybridization at 1dpf (D and D′) showing robust expression in several midline structures at 1dpf including the floor plate, chordamesoderm/notochord, and hypochord. At 2dpf (E and E′), the expression is specific to the large vacuolated cells. (F) Structure of the predicted 711-amino acid zebrafish Col8a1a protein. The N-terminal NC2 domain and C-terminal NC1 domain flank the more-canonical collagenous domain. The NC2 domain is thought to be a propeptide domain lost during maturation, whereas the collagenous and NC1 domains are important for trimerization and supermolecular network formation respectively. The G309E (levm531) and G365S (levvu105) substitutions are located within the collagenous domain (gray box); the 607 nonsense mutation (levvu41) resides just N-terminal to the NC1 domain and the previously described gulm208a mutation. PHENOTYPE:
|
|
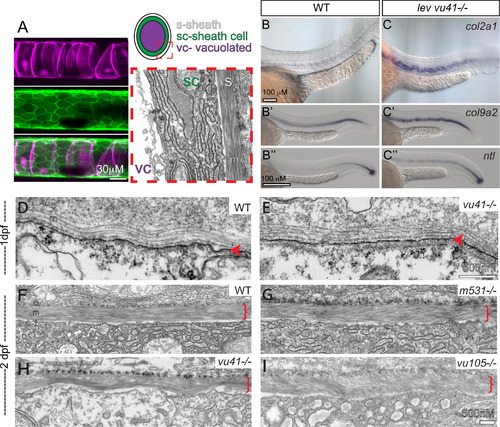
col8a1a mutations disrupt the normal morphology of the notochord sheath. (A) The primary components of the notochord tissue at embryonic maturation illustrated with a confocal (A, B, and C), cartoon (D), and transmission electron micrographs (TEM) (E). The large vacuolated cell layer (VC; Tg(TK5xC twhh:memRFP) expression; magenta), surrounded by a sheath epidermis (SC; 1.75KB col2a1 GFP-CAAX; green) are shown in a lateral view with a confocal projection. The cartoon and the TEM image are transverse views highlighting the extracellular tri-laminar notochord sheath (S) composed of laminin-, collagen- and elastin-rich components. (B–C′′) Representative examples of in situ hybridization of chordamesodermal genes col2a1 (B and C), col9a2 (B′ and C′), and ntl (B3, C3) in WT (B–B′′) and levvu41 mutant (C–C′) embryos. (C) The expression of col9a2 and ntl transcripts is upregulated, especially in more anterior regions of the notochord (C′ and C′′) as compared to time matched sibling embryos (B′ and B′). TEM of transverse sections from WT and mutant embryos at 2dpf (D–I). (D) WT notochord at 1 dpf. (E) levvu41-/- notochord at 1dpf. (F) WT notochord sheath at 2dpf display very straight, well organized medial sheath layer. Extracellular notochord sheaths of lev m531-/- (G), levvu41-/- (H), and lev vu105-/- (I) mutants exhibiting a wavy, disordered organization of the medial layer at 2dpf. I – inner laminin-rich layer, m – medial, and o – outer collagen-rich layers of the extracellular sheath; SC – sheath cell, S – extra-cellular sheath, and VC – vacuolated cell. |
|
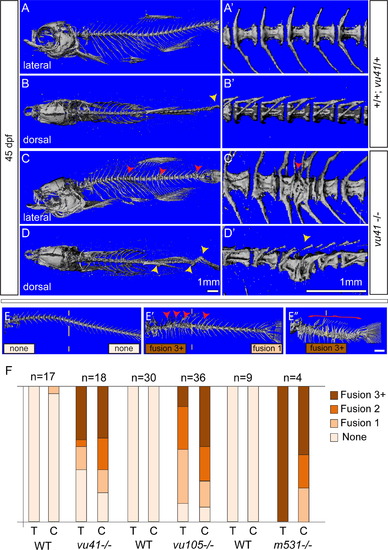
Homozyogus col8a1a mutant adults display vertebral fusions and scoliosis. MicroCT imaging of WT (A–B′) and col8a1a mutant zebrafish (C–D′) adults (45dpf) (A–E′′) in both lateral views (A, C, E–E′′) with insets (A′ and C′) and dorsal views (B and D) with insets (B′, D′) (A, A′, and B′) WT vertebrae with one neural and hemal arches per vertebral body. (B) Representative scoliosis in the tail of a WT adult (yellow arrowhead). (C and C′) Representative levvu41-/- zebrafish with VM (red arrowheads). (D and D′) Representative levvu41-/- multiple scoliosis along the axis (yellow arrowheads) and (E–E′′) Representative individuals scored for the presence of individual VM (red arrowheads) or complete fusion of vertebral column (red bracket) in the thoracic or caudal regions (as number of fused regions). (F) Graphed categorical data of VM from all lev alleles and unaffected WT siblings in thoracic (T) and caudal (C) regions. All scale bars (A–D), (A′–D′), and (E–E′′)=1 mM. PHENOTYPE:
|
|
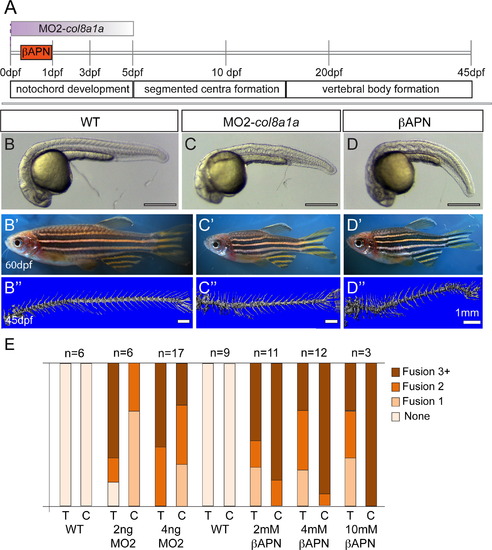
Embryonic inhibition of col8a1a function or lysyl oxidases is sufficient to generate vertebral defects. (A) Timeline of vertebral column development in zebrafish. In one experiment, the injection of MO2-col8a1a at the 1-cell stage, is effective in blocking the splicing of col8a1a until~3–5dpf (purple gradient bar) (C–C′′). In the second experiment the lysyl oxidases inhibitor, βAPN, is added at 4hpf and washed out at 1dpf (orange bar) (D–D3). Both treatments completed well before the onset of centra formation started in the anterior regions at 5dpf. Representative brightfield images of 1dpf (B, C, and D) and 60dpf (B′, C′, D′) and microCT images (45dpf) (B′′, C′′, D′′) of WT (B–B′′), MO2-col8a1a injected (C–C′′), and βAPN pulsed adult zebrafish (D–D′′). (D) Categorical frequency of VM for 2 and 4 ng MO2-col8a1a morpholino injected adults and uninjected WT siblings and 2, 4, and 10 mM βAPN treated adults and DMSO treated sibling controls for both thoracic (T) and caudal (C) regions. Scale bars for (B, C, and D)=4 mm, (B′, C′, D′)=5 mm, and (B′′, C′′, D′′)=1 mm. PHENOTYPE:
|
|
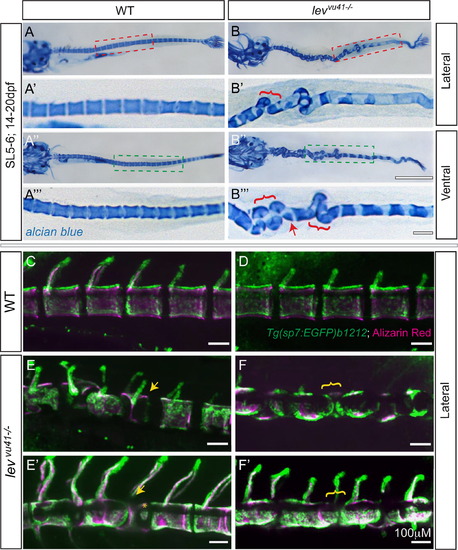
Segmentation of the vertebral column and localization of osteoblasts proceeds on a dysmorphic notochord template. Alcian blue stained larvae (14dpf) (A–B′′′) with lateral insets (red dashed box; A′, B′) and ventral insets (green dashed box; A′′′, B′′′). (A–A′′′) WT larvae display well-formed and segmented centra. (B–B′′′) levvu41-/- larvae display abberant apposition of centra (red brackets) and hemicentra (red arrow). Confocal projections of zebrafish vertebral column at 21dpf, illustrating boney matrix (alizarin red in magenta) and osteoblasts (Tg(sp7:EGFP)b1212 in green) (C–F′). (C, D) WT vertebrae and associated osteoblasts are well segmented along the vertebral axis. (E, E′) An individual levvu41-/- mutant displaying segmented colocalization of osteoblast and centra, including malformed hemivertebrae (yellow arrows) that continues to have mostly well segmented osteoblasts (E′), excepting some ectopic osteoblast-mineral formations in regions previously devoid of osteoblasts or mineral (E′, yellow asterix). (F and F′) An individual levvu41-/- mutant displaying regions of severe notochord bending with aberrantly apposed osteoblasts (F, yellow bracket), those progresses to a region with more complete osteoblast coverage (F′, yellow bracket). (C,D) 10dpf; ~SL3-4 (E,F) 10dpf; ~SL3-4 (E′, F′) 14dpf; ~SL4-5. Scale bars for (A, B, A3, B3)=1 mM; (C–F′) and (A′, A′′′,B′,B′′′)=100 mM. EXPRESSION / LABELING:
PHENOTYPE:
|
|
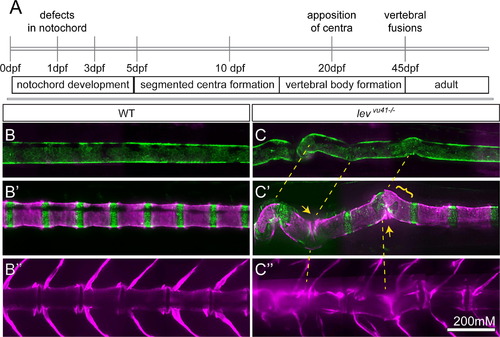
Periodic imaging of individual col8a1a mutants illustrates the progression of notochord bends towards malformed vertebral units. (A) Timeline of vertebral column development in zebrafish highlighting the timing of this particular periodic imaging method. Confocal projections of an transgenic individuals WT (B-B′′) or col8a1a/levvu41-/- mutant (C–C′′), and vitally stained with alizarin red (Tg[1.75KB col2a1 GFP-CAAX]; green (B and C′) and Alizarin Red; magenta (B–C′′) periodically imaged over the range of vertebral column development. (B, C) mature notochord (10dpf; ~SL 3.5–4) (B′ and C′) segmentation of axial centra (20dpf; ~SL 5–6) (B′′, C′′) mature vertebral column (39dpf; ~SL 8–9). (C) lev mutant individual at 10dpf displaying subtle bends in the notochord which transition to larger bends (yellow dashed lines (B′)). (C′) At 20dpf, these bends can display dysmorphic centra morphology (yellow bracket) or close apposition of centra edges in regions of severe bending (yellow arrows). (C′′) At 39 dpf, the formation of VM and scoliosis is predicted from the previous disrupted notochord regions (yellow dashed line). Scale bar is 200 mM. EXPRESSION / LABELING:
PHENOTYPE:
|
|
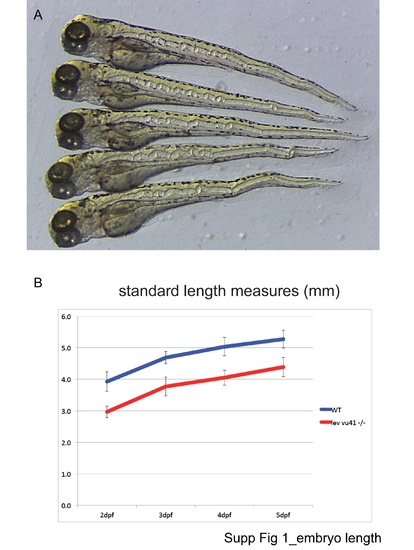
Embryo length. |
|
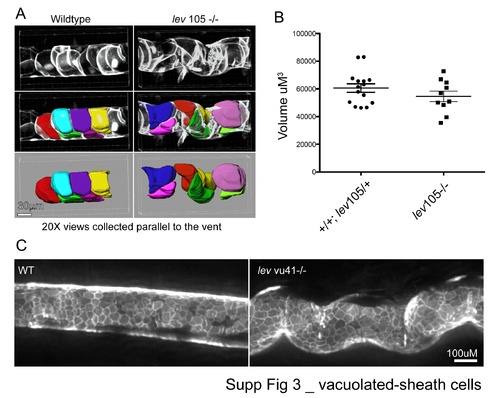
Vacuolated-sheath cells. |
|
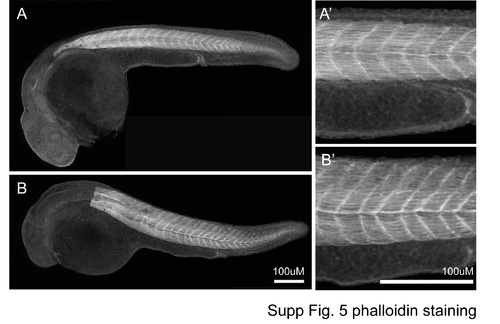
Phalloidin staining. |
Reprinted from Developmental Biology, 386(1), Gray, R.S., Wilm, T.P., Smith, J., Bagnat, M., Dale, R.M., Topczewski, J., Johnson, S.L., and Solnica-Krezel, L., Loss of col8a1a function during zebrafish embryogenesis results in congenital vertebral malformations, 72-85, Copyright (2014) with permission from Elsevier. Full text @ Dev. Biol.