- Title
-
neb: a zebrafish model of nemaline myopathy due to nebulin mutation
- Authors
- Telfer, W.R., Nelson, D.D., Waugh, T., Brooks, S.V., and Dowling, J.J.
- Source
- Full text @ Dis. Model. Mech.
|
Characterization of neb zebrafish. (A) DNA sequencing of neb adult carriers revealed a heterozygous sequence change (G>A) at IVS43+1 (**). (B) Live visualization of neb embryos and control (WT) littermates at 2 dpf. neb embryos are smaller and have thin bodies and tails. Genotyping confirmed that phenotypically abnormal embryos were homozygous for the G>A sequence change. (C) RT-PCR using exon 42–44 spanning primers of RNA extracted from pools of WT and neb embryos. WT fish have 2 major bands (arrowheads), a middle band that corresponds to the predicted size of exons 42–44 and a higher molecular weight band. neb zebrafish have diminished expression of these bands and instead have increased expression of a small band (**) with a size equal to that of exons 42–44 and excluding exon 43. Sequencing of this band confirmed that it lacked exon 43. (D) Western blot analysis of protein extracted from WT and neb embryos. Upper blot: with an anti-N-terminal Nebulin antibody, WT embryos had a single dominant band of very high molecular weight. This band was absent from neb embryos (arrow). Lower blot: confirmation of protein sample integrity and equal loading using anti-GAPDH antibody. |
|
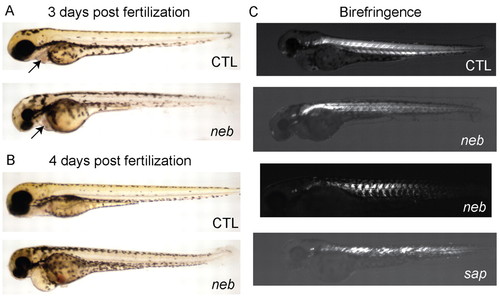
neb embryos are morphologically abnormal and have disrupted birefringence. Live imaging of neb embryos and age-matched littermates (CTL) at 3 dpf (A) and 4 dpf (B). Compared with controls, neb zebrafish are smaller, have thin and bent tails, and have pericardial edema. The heart structure is not obviously abnormal (arrows and see supplementary material Movies 3, 4). (C) Live image analysis of 3 dpf embryos using polarized light to detect birefringence. Wild type (CTL) embryos have normal birefringence, whereas neb embryos have diminished and patchy birefringence. The pattern is variable between neb embryos. Depicted for comparison is an age-matched sapje (sap) embryo. sap zebrafish have a recessive mutation in dystrophin and abnormal birefringence. PHENOTYPE:
|
|
Muscle contractions of neb larvae generate less force than wild-type larvae. Force generation was measured during a bilateral contraction of whole neb and wild-type larvae at 3 dpf (n=5). (A) Representative force response from neb and wild-type larvae. (B) Peak force normalized to cross-sectional area (CSA) was significantly less in neb larvae compared with wild type (4.0±2.0 mN/mm2 vs 20±1.7 mN/mm2, P<0.001). Data expressed as mean ± s.e.m. PHENOTYPE:
|
|
Altered thin filament length in skeletal muscle from neb embryos. (A) Ultrastructural analysis of neb embryos and wild-type clutchmates at 3 dpf. Thin filaments (I bands; marked with red arrows) are qualitatively smaller in neb embryos compared with wild type. Overall sarcomere length is also reduced (white arrows). Scale bar: 500 nm. (B) Co-immunostaining of isolated myofibers with antibodies to α-actinin (red), to mark the Z-lines, and tropomodulin (green), to mark the ends of the thin filaments. Note that α-actinin staining is somewhat diminished in neb embryos and that there are areas of disorganization of tropomodulin staining (arrow). Scale bar: 10 μm. (C) Quantification of thin filament (TF) length. TF length was calculated using electron micrographs by measuring from the Z-line to the beginning of the H zone. TF length in neb zebrafish was significantly reduced (in nm) compared with controls (CTL): 794.3±5.7 vs 603.8±7.9, n=4 averaged measurements, P<0.0001. PHENOTYPE:
|
|
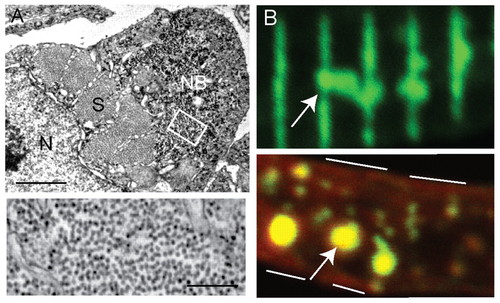
Nemaline bodies in neb skeletal muscle. (A) Ultrastructural analysis reveals the presence of aggregates similar to nemaline bodies. Top panel: a typical example of a nemaline body (NB) is depicted in a myofiber examined in cross-section. S, sarcomere; N, nucleus. Scale bar: 1 μm. Bottom panel: higher magnification of the area denoted by the white box demonstrates that the aggregates are composed primarily of filamentous material. Scale bar: 250 nm. (B) Immunohistochemical analysis of isolated myofibers from 3-dpf neb embryos using an antibody to α-actinin. Top panel: frequent areas of aberrant α-actinin accumulation were detected (arrow), consistent with the presence of nemaline bodies. Bottom panel: co-staining with phalloidin (red) reveals the presence of actin in the aggregated areas (arrow). White lines denote the sarcolemmal membrane. PHENOTYPE:
|

Unillustrated author statements PHENOTYPE:
|