- Title
-
One for all-a highly efficient and versatile method for fluorescent immunostaining in fish embryos
- Authors
- Inoue, D., and Wittbrodt, J.
- Source
- Full text @ PLoS One
|
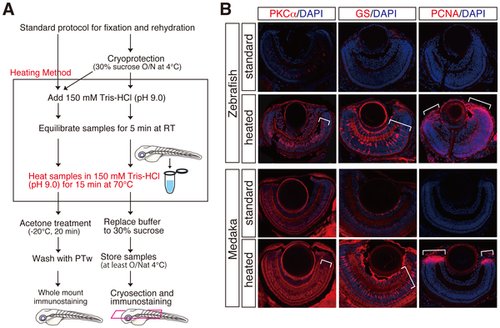
Improvement of fluorescent immunostainings by a novel heating method. (A) Schematic flowchart including a novel heating step in the standard immunostaining for cryosections and whole mount preparations. Whole mount and cryoprotected fish embryos were heated at 70°C for 15 min in 150 mM Tris-HCl at pH 9.0 (framed), subjected to the respective standard protocol for immunostaining. Cryoprotected preparations also can be used for whole mount immunostaining (bifurcated arrows). (B) Fluorescent immunostainings with cryosections of zebrafish and medaka retinae using anti-PKCα, anti-GS and, anti-PCNA antibodies. PKCα, GS, and PCNA immunostainings mark bipolar, Mueller glia, and retinal progenitor cells, respectively. Note that the heating method (heated) but not standard method (standard) efficiently improved all the immunostainings (lower panels, see brackets). Nuclei were counterstained with DAPI (blue). |
|
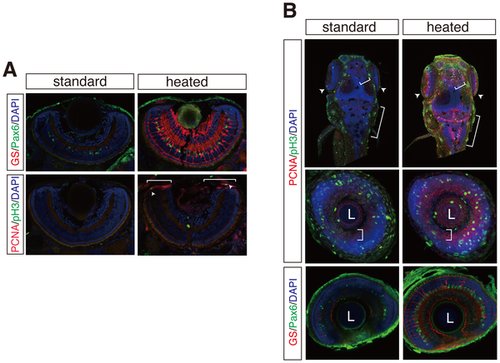
Multiple fluorescent immunostaining of cryosections and whole mount embryos by the heating method. (A) GS/Pax6 (top panels) and PCNA/phospho-histone H3 (pH 3) (bottom panels) immunostainings on cryosections of zebrafish retinae. GS and Pax6 immunostainings detect amacrine and Mueller glia cells. pH 3-positive mitotic cells (arrowheads) co-localize to PCNA-positive retinal progenitor cell region (brackets) in the ciliary marginal zone (CMZ). (B) Whole mount fluorescent immunostainings as in A with or without the heating method. Maximum projection of dorsal view of whole mount zebrafish (top panels). Coronal optical sections of zebrafish retina (middle and bottom panels). Brackets and arrowheads indicate proliferative zones of hindbrain, optic tectum, telencephalon, and CMZ. L; lens. Nuclei were counterstained with DAPI (blue). |
|
Application of heating method to co-staining with fluorescent proteins (FPs) in transgenic lines. (A) PCNA whole-mount immunostaining with Ath5::GFP medaka transgenic line. (B) Zn5 whole-mount immunostaining with Ath5::GAP43-RFP zebrafish transgenic line. Note that Zn5 overlaps with RFP-positive optic nerve and retinal progenitor cells (brackets) (C) GS and PCNA immunostainings on cryosections of Rx2::H2B-RFP medaka transgenic line. Rx2 dependent H2B-RFP expression clearly co-localizes with GS-positive Mueller glia cells (arrowheads) and PCNA positive retinal progenitor cells in the CMZ (brackets). Nuclei were counterstained with DAPI (blue). |
|
Comparable and improved fluorescent immunostainings of cryosections by the heating method. (A) Comparable fluorescent immunostainings of cryosections with or without the heating method in zebrafish and medaka. Note that the heating method as well as standard protocol fully preserved the retinal morphology. (B) Immunostainings improved by the heating method. HuC/D (bracket), phospho-histone H3 (pH 3) (arrowheads), and GFAP (brackets) immunostainings were strongly improved as compared to standard protocol in both zebrafish and medaka. (A) and (B) Rhodopsin (brackets) and Recoverin; photoreceptor cell layer. Pax6; amacrine cells. Islet1; retinal ganglion and neuronal cells in the inner nuclear layer. HuC/D; retinal ganglion and amacrine cell layers. pH 3; mitotic retinal progenitor cells. GFAP; Mueller glia cells. Nuclei were counterstained with DAPI (blue). |
|
Application of heating method to co-staining with fluorescent proteins (FPs) in transgenic lines and whole-mount in situ hybridization. (A) HuC/D and pH 3 immunostainings of cryosections with Ath5::GFP medaka transgenic line. The heating method sufficiently retained GFP fluorescence after heating. GFP-positive retinal ganglion cells co-stained with HuC/D-positive retinal ganglion cells (a bracket) and pH 3-positive mitotic dividing cells (arrowheads). (B) HuC/D immunostaining of cryosections with Ath5::GAP43-RFP (RFP) zebrafish transgenic line. Note that fluorescent signal was still strong enough to detect after heating. (C) Whole mount in situ hybridization of medaka by uing antisense Rx2 probe in combination with either GS or PCNA immunostaining. Note that Rx2 co-stained with GS-positive Mueller glia cells (arrowheads) and PCNA-positive retinal progenitor cells at CMZ (brackets). Insets denote higher magnification of the overlap regions. Nuclei were counterstained with DAPI (blue). |