- Title
-
Evx1 is required for joint formation in zebrafish fin dermoskeleton
- Authors
- Schulte, C.J., Allen, C., England, S.J., Juárez-Morales, J.L., and Lewis, K.E.
- Source
- Full text @ Dev. Dyn.
|
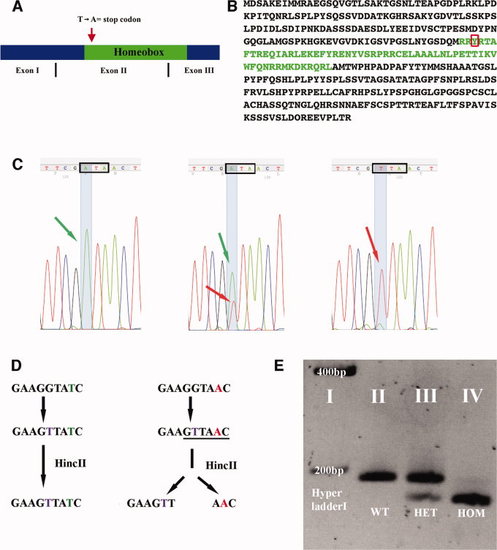
evx1 mutation probably causes a complete loss of function. A: Schematic of evx1 cDNA indicating exon boundaries. Red arrow shows approximate position of point mutation that converts a Thymine to Adenine and, hence, the Tyrosine (red box in B) into a stop codon. B: Amino acid sequence of Evx1, homeobox in green. C:evx1 sequencing results (reverse complement) for wild-type (WT; left), heterozygous (middle) and homozygous mutant (right) zebrafish. Green arrows indicate Thymine (reverse complement = A; WT allele); Red arrows indicate Adenine (reverse complement = T; mutant allele). Black box outlines the codon that produces Tyrosine in WTs and an ochre stop codon in mutants. Note the change in sequence (SYR [AGT TAT CGA] instead of RYR [AGG TAT CGA]) deliberately introduced by mismatch primers during polymerase chain reaction (PCR; see the Experimental Procedures section). D: Schematic of evx1 mutant genotyping. WT allele left, mutant right. Thymine (purple) is substituted for Guanine by means of PCR. This creates a HincII site (underlined) in mutant DNA. Subsequent enzymatic digestion results in two fragments (158 bp and 28 bp) in mutants while leaving WT DNA uncut (186 bp). E: Genotyping results visualized by gel electrophoresis. The 28-bp fragment generated by HincII digest of mutant DNA is not visible. PHENOTYPE:
|
|
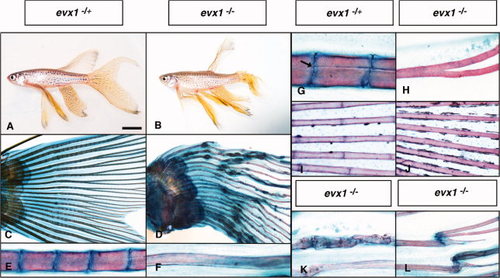
Evx1 is required for joint formation in dermoskeleton but not bony ray bifurcation. A–L: Lateral views of heterozygous sibling (A,C,E,G,I) and evx1 mutant (B,D,F,H,J–L) adult zebrafish (A–H,K,L) and 28 days postfertilization (dpf) larvae (I,J). A–F: Adult zebrafish in the TL background (which includes the longfin mutation). For a similar analysis in the AB background, please see Supp. Figure S1. C–L: Alcian blue/Alizarin red staining of caudal fins. Anterior left and dorsal top. B,D: evx1 mutants still form fins, but these fins often exhibit structural damage. F,J: evx1 mutants lack dermoskeletal joints. Size differences in (E) and (F) reflect different ray thicknesses along the proximo-distal fin axis. I,J: By 28 dpf, WT larvae have developed 4–5 joints (I) while joints are completely absent in mutants (J). H: Fin ray bifurcations still form in evx1 mutants. G: Note that bifurcations of WT rays start at joints (arrow). K,L: Bone fractures and irregular bone structures in evx1 mutants. Scale bar = 1.2 cm in A,B, 1 mm in C,D, 140 μm in E,F, 100 μm in G–J, 200 μm in K, 260 μm in L. |
|
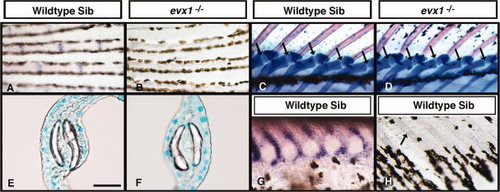
Endoskeletal joints and hemisegment separation do not require Evx1. A–D,G,H: Lateral views of wild-type (WT) or heterozygous siblings (A,C,G,H) and evx1 mutants (B,D). E,F: Cross sections through WT (E) and evx1 mutant (F) lepidotrichia. A,B,G,H: Anal fins. C,D: Dorsal fins. E,F: Caudal fins. A,B: 43 days postfertilization (dpf). C,D: 28 dpf. E,F: Adult. G: 22 dpf. H: 35 dpf. A,B: evx1 expression. C–F: Alcian blue/Alizarin red staining. G,H: gdf5 expression. A,B: evx1 is expressed at intersegmental joints in WT siblings (A), but it is not expressed in equivalent regions in evx1 mutant fins (B). Fin endoskeleton is not affected by the evx1 mutation. C,D: The distal radials of dorsal fins separate from the proximal radials (arrows) in both WT siblings (C) and evx1 mutants (D). E,F: Dermoskeletal hemisegments are separated and not fused in WT (E) and evx1 mutant (F) fins. G,H: gdf5 is expressed during joint formation in the endoskeleton (G) but not in the dermoskeleton (H). Arrow in H indicates a joint clearly visible by differential interference contrast microscopy. Scale bar in panel E = 120 μm in A,B, 160 μm in C,D, 34 μm in E,F, 55 μm in G, 100 μm in H. EXPRESSION / LABELING:
|
|
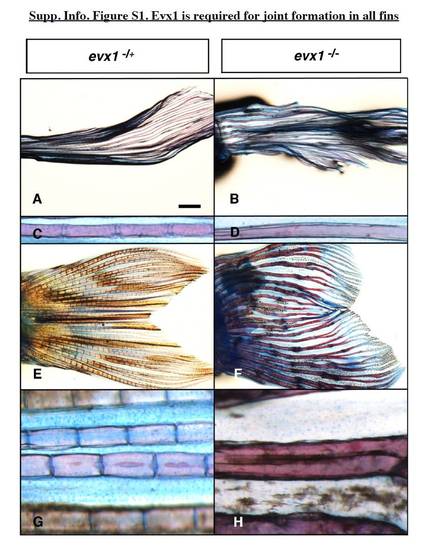
Evx1 is required for joint formation in all fins. A–H: Lateral views of Alcian blue/Alizarin red staining in wild-type (WT) or heterozygous siblings (A,C,E,G) and evx1 mutant (B,D,F,H) adult zebrafish. Anterior is left. A–D: Pectoral fins in the TL background. E–H: Caudal fins in the AB background. Scale bar = 1.4 mm in A,B, 100 μm in C,D, 1 mm in E,F, 64 μm in G,H. |