- Title
-
Zebrafish retinoic acid receptors function as context-dependent transcriptional activators
- Authors
- Waxman, J.S., and Yelon, D.
- Source
- Full text @ Dev. Biol.
|
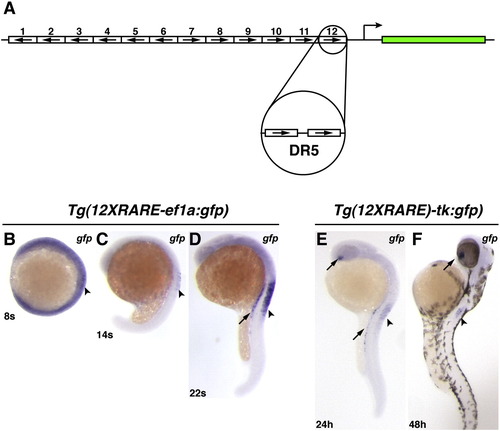
Zebrafish RARE transgenic reporter lines. (A) Schematic representation of the 12XRARE reporters. Arrows indicate direction of each DR5 RARE site. (B) At 8 somites, the expression of gfp from Tg(12XRARE-ef1a:gfp) can be seen at low levels in the anterior spinal cord (arrowhead). At this stage, embryos require a much longer exposure to visualize gfp expression than at subsequent stages. (C) At 14 somites, gfp expression is more easily detected in the spinal cord (arrowhead). (D) By 22 somites, the reporter is expressed at higher levels in the pronephros (arrow) and spinal cord (arrowhead). Shortly afterward, the reporter is also expressed in the ventral eye (not shown), similar to Tg(12XRARE-tk:gfp). (E) Expression of Tg(12XRARE-tk:gfp) does not initiate until closer to 24 hpf, when it is seen in the ventral eye (upper arrow), pronephros (lower arrow), and the anterior spinal cord (arrowhead). (F) Expression of Tg(12XRARE-tk:gfp) in the eye (arrow) and spinal cord (arrowhead) is maintained past 48 hpf. All images are lateral views, with anterior up and dorsal to the right. |
|
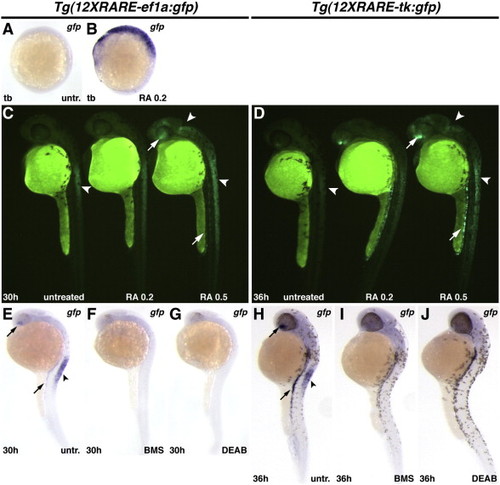
Zebrafish RARE transgenic reporter lines require and are responsive to RA signaling. (A) Untreated Tg(12XRARE-ef1a:gfp) embryo. (B) Treatment of Tg(12XRARE-ef1a:gfp) embryos with 0.2 μM RA induces reporter expression. (C, D) Tg(12XRARE-ef1a:gfp) and Tg(12XRARE-tk:gfp) embryos respond to treatment with RA for 6 h beginning at 24 hpf (C) or 30 hpf (D). Untreated sibling embryos (left) exhibit expression in the anterior spinal cord (arrowhead), the ventral eye, and pronephros. Treatment with 0.2 μM RA (center) causes a more modest response than does treatment with 0.5 μM RA (right). Increased fluorescence is induced in the anterior brain/eye (upper arrow), midbrain and spinal cord (arrowheads) and pronephros (lower arrow). (E, H) Untreated Tg(12XRARE-ef1a:gfp) (E) and Tg(12XRARE-tk:gfp) (H) embryos, respectively. Treatment with RA signaling antagonists BMS (F, I) and DEAB (G, J) inhibit expression of Tg(12XRARE-ef1a:gfp) and Tg(12XRARE-tk:gfp). Embryos were treated with RA signaling antagonists for 6 h beginning at 24 hpf (E–G) or 30 hpf (H–J). All images are lateral views, with anterior up and dorsal to the right. |
|
Ectopic expression of zebrafish RARs does not sensitize embryos to RA signaling. (A) Schematic composition of RAR constructs used in experiments. (B) Uninjected Tg(12XRARE-ef1a:gfp) embryo. (C, D) Tg(12XRARE-ef1a:gfp) embryos injected with zebrafish rarab (C) or rarga (D) mRNA appear normal, and reporter expression is unaffected. (E) Tg(12XRARE-ef1a:gfp) embryo continuously treated with 0.05 μM RA. (F, G) Tg(12XRARE-ef1a:gfp) embryo injected with zebrafish rarab (F) or rarga (G) mRNA and treated with 0.05 μM RA are not more affected than uninjected embryos. All images are lateral views, with anterior up and dorsal to the right. |
|
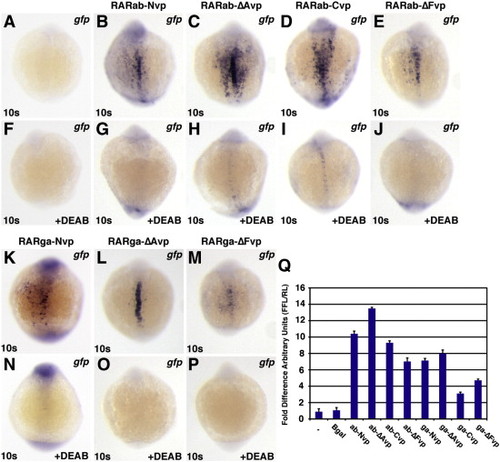
RAR-vps require RA to function. All images depict Tg(12XRARE-ef1a:gfp) embryos. (A, F) Uninjected control embryos. (B–E, K–M) Injection with (B) rarab-Nvp, (C) rarab-ΔAvp, (D) rarab-Cvp, (E) rarab-ΔFvp, (K) rarga-Nvp, (L) rarga-ΔAvp, or (M) rarga-ΔFvp mRNA induces ectopic reporter expression. (F–P) Treatment with 1 μM DEAB beginning at 40% epiboly blocks reporter expression in injected embryos. All images are dorsal views with anterior up. (Q) In HEK 293 cells transfected with the pGL3-basic:12XRARE-tk reporter, co-transfection with RAR-vp constructs caused similar trends of reporter activation to those found in zebrafish embryos. Arbitrary Units (AU) represents the ratio of firefly luciferase to Renilla luciferase. Each column represents the average AU plus standard deviation (bars) of 3 replicates for a condition from one representative experiment. Equivalent trends were observed using the pGL3-basic:12XRAR-ef1a reporter; additionally, DEAB treatment of cells transfected with RAR-vp constructs reduced reporter activation (JSW and DY, data not shown). |
|
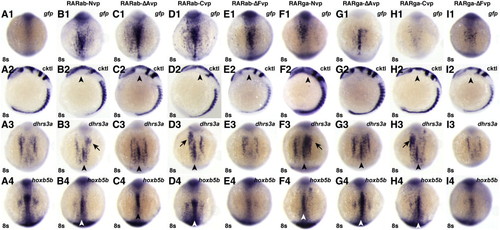
RAR-vps can induce ectopic expression of RA target genes. (A1–I1) Injection of the rar-vp mRNAs differentially activates reporter expression. (A2–I2) Injection of the rar-vp mRNAs, except for rarga-ΔAvp, eliminates the MHB. Cocktail (cktl) of probes includes opl/zic1 (telencephalon, diencephalic-midbrain boundary), eng2a (MHB), krox20/egr2b (rhombomeres 3 and 5), and myod (somites and adaxial cells). Arrowheads indicate loss of enr2 expression in the MHB. (A3–I3, A4–I4) Injection of the rarNvp, rarΔAvp, and rarCvp mRNAs can induce ectopic expression of the RA signaling target genes dhrs3a and hoxb5b, while injection of the rarΔFvp mRNAs inhibits their expression. Arrowheads indicate ectopic dhrs3a or hoxb5b expression in the notochord. Arrows indicate ectopic dhrs3a expression more anteriorly. (A1–A4) Control uninjected embryos. (B1–B4) rarabNvp mRNA-injected embryos. (C1–C4) rarabΔAvp mRNA-injected embryos. (D1–D4) rarabCvp mRNA-injected embryos. (E1–E4) rarabΔFvp mRNA-injected embryos. (F1–F4) rargaNvp mRNA-injected embryos. (G1–G4) rargaΔAvp mRNA-injected embryos. (H1–H4) rargaCvp mRNA-injected embryos. (I1–I4) rargaΔFvp mRNA-injected embryos. All images except A2–I2 are dorsal views with anterior up. Images in A2–I2 are lateral views with dorsal to the right. |
|
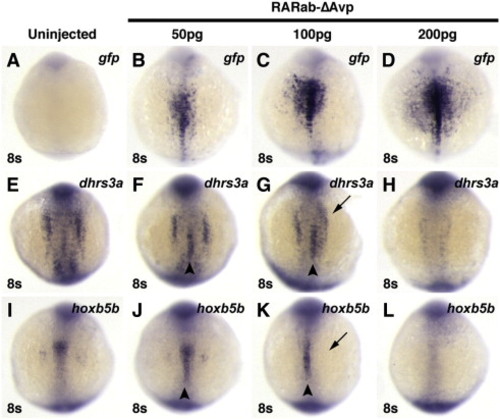
Injection of high doses of hyperactive RAR mRNA can inhibit target gene expression. Injection of increasing concentrations of rarabΔAvp mRNA increasingly activates reporter expression (A–D), but inhibits endogenous target gene expression (E–L). (A, E, I) Uninjected control embryos. (B, F, J) In embryos injected with 50 pg of rarabΔAvp mRNA, modest levels of the reporter are induced (B), as well as ectopic expression of dhrs3a and hoxb5b (arrowheads in F and J). (C, G, K) In embryos injected with 100 pg of rarabΔAvp mRNA, higher levels of the reporter are induced (C). Although ectopic activation of dhrs3a and hoxb5b is induced (arrowheads in G and K), their endogenous expression is also inhibited (arrows in G and K). (D, H, L) In embryos injected with 200 pg of rarabΔAvp mRNA, the highest levels of the reporter are induced (D), while endogenous dhrs3a and hoxb5b expression are inhibited and ectopic expression is not induced (arrowheads in H and L). All images are dorsal views with anterior up. |
|
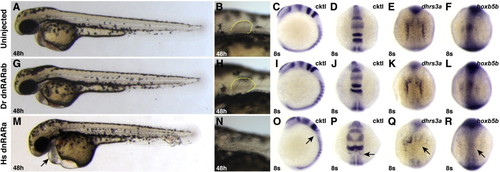
Zebrafish dnRARab does not act as a transcriptional repressor in zebrafish embryos. (A–F) Uninjected control embryos. (G–L) Embryos injected with zebrafish dnrarab mRNA appear equivalent to uninjected control embryos. (M–R) Injection of human dnrara mRNA causes phenotypes consistent with loss of RA signaling, including enlarged hearts (arrow in M), loss of pectoral fin (N), loss of rhombomere 5 (arrows in O and P), and loss of endogenous target gene expression (arrows in Q and R). Phenotypes induces by injection of the human dnrara mRNA are similar to, if not stronger than, those induced when the human dnRARa is expressed from a heat-shock inducible transgene (Waxman et al., 2008). Cktl of probes is the same as in Fig. 6. (A–C, G–I, M–O) Images are lateral views with dorsal to the right. (D–F, J–L, P–R) Images are dorsal views, anterior up. |
|
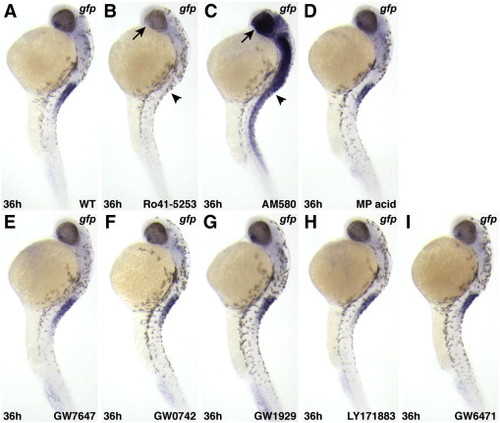
RAR-specific pharmacological reagents affect expression of Tg(12XRARE-ef1a:gfp), while pharmacological reagents specific for other nuclear hormone receptors do not. (A) Untreated control embryo. (B) Ro41-5253, a RARα-specific antagonist, inhibits reporter expression. (C) AM580, a RARα-specific agonist, activates ectopic reporter expression. Arrows (ventral eye) and arrowheads (anterior spinal cord) in B and C indicate where restricted expression is normally found in an untreated embryo (A). (D) The pan-RXR agonist methoprene acid (Biomol.com) does not affect reporter expression. Agonists of (E) PPARα (GW7647), (F) PPARδ (GW0742), and (G) PPARγ (GW1929) do not activate the reporter. The pan-PPAR antagonist LY171883(H) and the PPARα specific antagonist GW6471 (I) do not inhibit reporter expression. All PPAR-related reagents were purchased from Sigma. All images are lateral views, with anterior up and dorsal to the right |
|
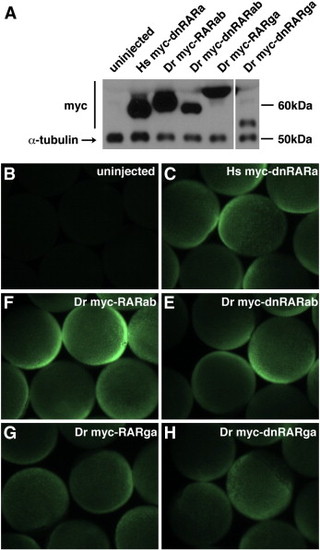
Myc-tagged zebrafish RARs are expressed in zebrafish embryos. (A) Western blot of myc-tagged Hs dnRAR and zebrafish WT RARs and dnRARs at 70–90% epiboly in zebrafish embryos that were injected with the indicated mRNAs. The decreasing relative expression from left to right reflects the timing of execution for this representative experiment, in which constructs on the left were injected earlier than those on the right. Approximate sizes of the myc-tagged proteins are: Hs myc-dnRARa, 55 kDa; Dr myc-RARab, 61 kDa; Dr myc-dnRARab, 55 kDa; Dr myc-RARga, 67 kDa; Dr myc-dnRARga, 52 kDa. The approximate size of α-tubulin is 50 kDa. (B–D) Wholemount immunofluorescence at 70–90% epiboly of zebrafish embryos that were injected with mRNAs for myc-tagged human and zebrafish RARs. Exogenous protein expression was analyzed at mid- to late-gastrulation stages because this is when RA signaling is required to pattern the early zebrafish embryo ([Hernandez et al., 2007], [Hernandez et al., 2004], [Linville et al., 2009], [Maves and Kimmel, 2005] and [Stafford et al., 2006]).Both analyses indicate that, while there may be differences in precise expression levels of different exogenous RARs, all of the exogenous RARs are expressed broadly and robustly in zebrafish embryos at these stages. Unfortunately, there are no commercially available anti-RAR antibodies that allow evaluation of the endogenous levels of zebrafish RARs. If we presume that endogenous α-tubulin is likely to be present at higher levels than endogenous RARs, the qualitative comparison of exogenous RARs and endogenous α-tubulin suggests that the exogenous RARs are present in excess of their endogenous counterparts. |
|
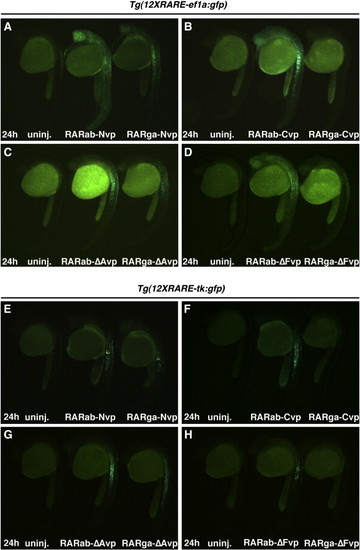
RARabvps and RARgavps differentially activate reporter expression in RARE transgenic reporter lines. (A–D) Ectopic expression of RAR-vps can induce ectopic expression of Tg(12XRARE-ef1a:gfp) at 24 hpf. (E–H) Ectopic expression of RAR-vps can induce ectopic expression of Tg(12XRARE-tk:gfp) at 24 hpf. Induction of expression by RARabvps (center in A–H) is typically stronger than that induced by RARgavps (right in A–H). Uninjected sibling embryos are at the left in A–H. All images are lateral views, with anterior up and dorsal to the right. |
|
Hyperactive RAR-vps induce regional activation of the RARE reporter. Tg(12XRARE-ef1a:gfp) embryos overexpressing RAR-vps. (A) Control uninjected embryos and embryos injected with (B) rarab-Nvp, (C) rarab-ΔAvp, (D) rarab-Cvp, (E) rarab-ΔFvp, (F) rarga-Nvp, (G) rargaΔAvp, and (H) rargaΔFvp mRNAs. Reporter expression is enhanced in regions of the embryo near a source of RA. Arrow in C indicates expanded ventral eye expression. Arrowhead in C indicates reporter expression within the trunk. All images are lateral views, with anterior up and dorsal to the right. |
|
Phenotypes induced by RA treatment. (A–C) RA treatment eliminates anterior neural gene expression. Cktl of probes is the same as in Fig. 6. (A,D,G) Untreated control embryos. (B) Treatment with 0.5 µM RA can strongly posteriorize embryos, resulting in loss of all anterior neural markers. (C) Treatment with 0.2 μM RA can yield moderately posteriorized embryos in which the MHB is lost and the expression of krox20 in rhombomere 3 is reduced. This more modest posteriorization is remniscent of rar-vp mRNA-injected embryos (Fig. 6), though the eyes of rar-vp mRNA-injected embryos are never lost. (D–I) RA signaling positively regulates expression of dhrs3a and hoxb5b. (E, H) Treatment with DEAB inhibits dhrs3a and hoxb5b expression. (F, I) Treatment with 0.5 μM RA induces ectopic dhrs3a and hoxb5b expression. Images in A–C are lateral views with dorsal to the right. All other images are dorsal views with anterior up. |
|
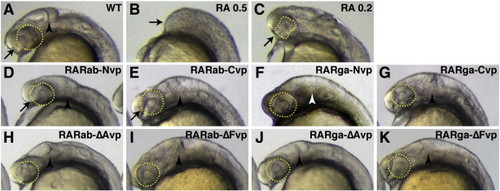
Ectopic expression of hyperactive RAR-vps does not phenocopy all aspects of RA treatment. (A) Uninjected control embryo. (B) Treatment with 0.5 μM RA causes loss of the anterior CNS (arrow). (C) Treatment with 0.2 μM RA causes dysmorphic, reduced eyes (arrow and dashed yellow outline) and loss of the MHB. Injection of (D) rarab-Nvp, (E) rarab-Cvp, (F) rarga-Nvp, (G) rarga-Cvp, (H) rarab-ΔAvp, (I) rarab-ΔFvp, and (K) rarga-ΔFvp mRNA can eliminate the MHB (arrowheads), but the eyes are not affected (arrows and dashed yellow outline). rarab-Nvp and rarab-Cvp mRNAs are particularly effective at eliminating the MHB. (J) Injection of rarga-ΔAvp mRNA does not affect the MHB (arrowhead |
|
Zebrafish dominant negative RARs and Enr fusion proteins do not inhibit expression of RA-responsive target genes. (A, F) Uninjected control embryos. (B–E, G–J) Injection of mRNAs for zebrafish dnrarga (B, G), dnraraa (C, H), rarab-ΔAenr (D, I) and rarab-ΔFenr (E, J) do not significantly affect expression of RA-responsive target genes. All images are dorsal views with anterior up. |
|
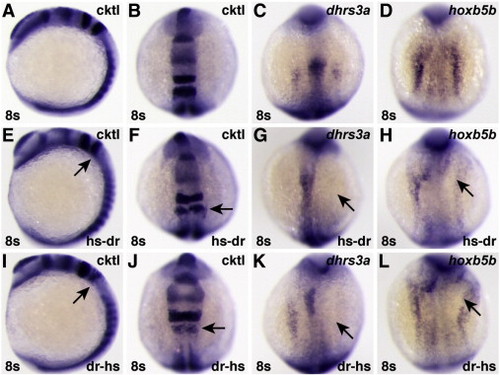
Chimeric human/zebrafish dominant negative RARs can inhibit expression of RA-responsive target genes. (A–D) Uninjected control embryos. (E–H) Embryos injected with mRNA encoding a hs-dr RAR fusion protein, in which the human A–C domains are fused to the zebrafish D–ΔF domains. (I–L) Embryos injected with mRNA encoding a dr-hs RAR fusion protein, in which the zebrafish A–C domains are fused to the human D–ΔF domains. Although neither chimeric protein was as effective as the human dnRARa (Figs. 8O–R), either can inhibit expression of RA-responsive genes. |
Reprinted from Developmental Biology, 352(1), Waxman, J.S., and Yelon, D., Zebrafish retinoic acid receptors function as context-dependent transcriptional activators, 128-140, Copyright (2011) with permission from Elsevier. Full text @ Dev. Biol.