- Title
-
Protocadherin-19 is essential for early steps in brain morphogenesis
- Authors
- Emond, M.R., Biswas, S., and Jontes, J.D.
- Source
- Full text @ Dev. Biol.
|
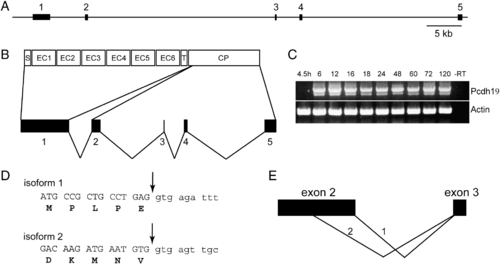
Genomic organization and alternative splicing of pcdh19. (A) The pcdh19 gene comprises 5 exons distributed over 85 kb of genomic sequence. (B) The entire extracellular domain, the transmembrane domain and a short segment of cytoplasmic domain are encoded by exon 1. The remainder of the cytoplasmic domain is encoded by exons 2–5. (C) RT-PCR reveals that expression of pcdh19 begins by 6 hpf and continues through development. In addition, we observe two splice variants: a larger, major band and a shorter minor band. There does not appear to be temporal variation in the expression of the two isoforms. (D) Cloning and sequencing of the two PCR products reveals that the major band represents the full-length form of Pcdh19; we term this variant “isoform 1.” The shorter band represents the use of an alternative splice site, internal to exon 2, that deletes ∼ 70 amino acids from the cytoplasmic domain; we refer to this variant as “isoform 2.”. E, Schematic representation of the choice of alternative donor sites in exon 2. |
|
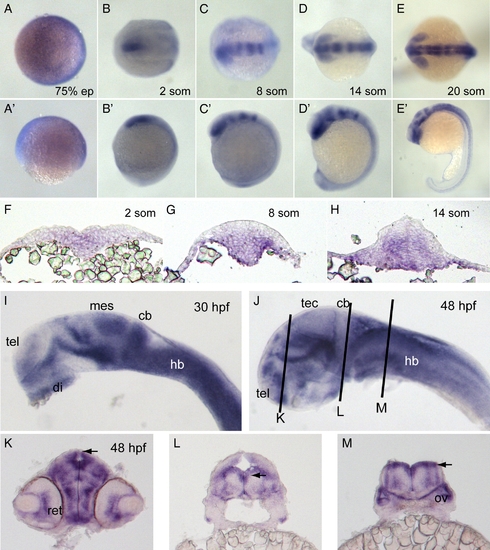
Expression of pcdh19. (A–E) In situ hybridization of embryos labeled with pcdh19 riboprobe against cadherin repeats 1–3 at 75% epiboly (A), 2 somites (B), 8 somites (C), 14 somites (D) and 20 somites (E). Dorsal views are shown in A–E and lateral views are shown in A′–E′. Pcdh19 is expressed broadly and diffusely at 75% epiboly. At 2 somite stage, pcdh19 is expressed in the medial anterior neural plate. By 8 somite stage, pcdh19 expression exhibits a banding pattern, with the anterior band encompassing the forebrain and eye primordia and additional bands present in the midbrain and hindbrain. These bands become sharper by 14 somite stage. By 20 somite stage, the pattern in the brain has become more complex, with expression in the eye, hypothalamus, ventral midbrain and the tectum. In addition, expression has become stronger and more uniform throughout the hindbrain. In the trunk region, pcdh19 is expressed in cells along the midline. F–H, Cross-sections of embryos labeled with pcdh19 riboprobe at 2 somite stage (F), 8 somite stage (G) and 14 somite stage (H). At 2 somite stage, pcdh19 is expressed medially in cells throughout the dorsoventral extent of the neural plate. As development proceeds, pcdh19 expression is concentrated more ventrally in both the neural keel and neural rod. I–J, In situ hybridizations reveal more distinctive expression patterns at 30 hpf (I) and 48 hpf (J). Abbreviations of brain regions are as follows: tel, telencephalon; di, diencephalon; mes, mesencephalon; cb, cerebellum; hb, hindbrain; tec, optic tectum. K–M, Cross-sections of 48 hpf embryos at the three planes of section indicated in (J). Both the retina (ret) and the otic vesicle (ov) are labeled with the pcdh19 riboprobe. Notably, the neuroepithelia that line the ventricles (arrowheads) express high levels of pcdh19. EXPRESSION / LABELING:
|
|
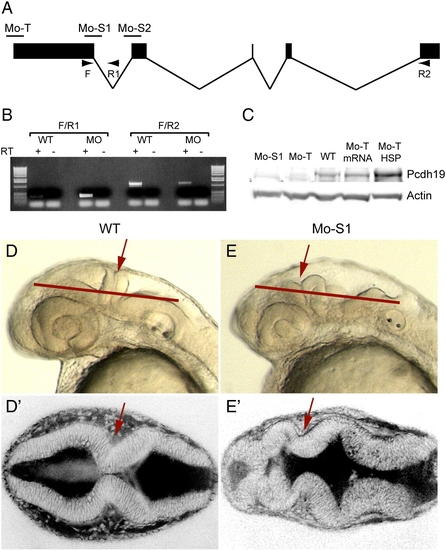
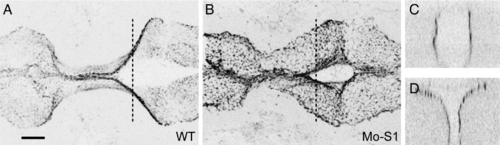
Protocadherin-19 is required for brain morphogenesis. (A) Antisense morpholino oligonucleotides were designed against pcdh19, including a translation-blocking (Mo-T) and two splice-blocking (Mo-S1 and Mo-S2) morpholinos. Diagnostic primers (arrows: F, R1 and R2) are shown. (B) Diagnostic RT-PCR was used to confirm the predicted effect of Mo-S1. Injection of Mo-S1 into 1-cell zebrafish embryos is predicted to result in the inclusion of intron 1 and a premature stop codon just downstream of the exon 1 splice-donor site. Wild type embryos show a strong band with F/R2 (normally spliced transcripts) and a very faint PCR product with F/R1, (unspliced or aberrantly spliced transcripts). The small amount of F/R1 PCR product may result from isolated pre-mRNA. In contrast, morphant embryos exhibit a strong band using the F/R1 primer set and reduced levels of F/R2 PCR product, indicating a shift toward aberrantly spliced pcdh19 transcripts. (C) Western blots using an affinity-purified rabbit polyclonal antibody against the Pcdh19 cytoplasmic domain shows a reduction in protein levels in extracts prepared from morphant embryos (Mo-S1 and Mo-T) compared to uninjected embryos (WT), and a recovery of Pcdh19 levels in morphants co-injected with mRNA encoding Pcdh19 (Mo-T/mRNA) or an HSP70:Pcdh19 plasmid (Mo-T/HSP). (D) Injection of Mo-S1 results in embryos with severely disrupted brain morphology. Shown here are lateral views of wild type (D) or morphant (D′) embryos at 30 hpf. In morphants, the hindbrain is bumpy, the MHB (red arrows) appears misfolded and the forebrain and midbrain are compressed anteriorly within the head. Red lines indicate the approximate plane of section of the images shown in E and E′. (E) Dorsal views of live embryos that have been labeled with the vital dye, BODIPY-ceramide. Images are single optical sections. Wild type embryos (D) exhibit a symmetrically folded neural tube, with expanded midbrain and hindbrain ventricles and a characteristic constriction at the MHB (red arrows). In contrast, the brain of morphant embryos appears misfolded (E′): the forebrain has ectopic folds and cell masses and appears compressed, and contact between lateral halves of the alar plate is not maintained at the midline of the MHB (red arrows). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Protocadherin-19 is required for early stages of morphogenesis in the anterior neural plate. A–C, Transmitted light images of live control and morphant embryos at 10–11 somite stage. Disruption of morphogenesis in the head is already disrupted. A–A″. In control embryos (injected with Mo-S1mis), the brain appears smooth in lateral views (A) and as a featureless rod in dorsal views (A′), as well as in face-on views (A″). The neural rod is highlighted with red shading. In morphant embryos, the brain is disorganized and the neural rod has the appearance of being misfolded or buckled (B, C) and the optic primordia are often malformed. In dorsal views, the neural rod is very disorganized and is much wider than control embryos (B′, C′). In face-on views, the neural rod is split, having a Y-shaped appearance (B″, C″). D–G, Shown are cross sections of 4 somite (D, E) and 10 somite (F, G) stage embryos that were labeled with DAPI. At 4 somite stage, the neural keel is wedge-shaped in the trunk and broader in both the posterior and anterior brain regions. The neural keel is comparable in morphant embryos (E–E″; Mo-S1), although it tends to be somewhat wider. At 10 somite stage, the neural keel of wild type embryos has converged at all levels along the antero-posterior axis (F–F″). In contrast, the neural keel in morphants (G–G″) has failed to converge in the posterior brain and appears disorganized in the anterior brain, although the trunk appears normal. PHENOTYPE:
|
|
Impaired of neural plate convergence in pcdh19 morphants. Shown are embryos labeled with riboprobe directed against foxd3, which defines the lateral boundaries of the neural plate. A–C, At 2 somite stage, the width of the neural plate is comparable between control embryos (A) and morphant embryos injected with splice-blocking (B; Mo-S1) or translation-blocking (C; Mo-T) morpholinos. By the 5 somite stage, the neural plate has converged substantially in control embryos (A′), but is much wider in morphants (B′ and C′). This impairment of convergence in morphants becomes even more pronounced by the 10 somite stage. The neural keel narrows somewhat in control embryos (A″), but little convergence appears to occur between 5 and 10 somite stages in morphant embryos (B″ and C″). PHENOTYPE:
|
|
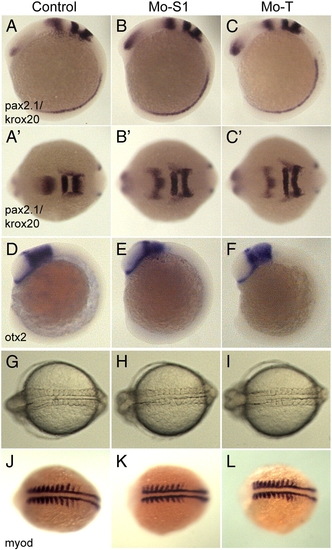
Disruption of pcdh19 function does not alter axial patterning or somitogenesis. A–C, To assess the effects of pcdh19 depletion on regional specification in the brain, we performed in situ hybridizations on 10 somite stage embryos using riboprobes for pax2.1, which labels the midbrain–hindbrain boundary, and krox20, which labels rhombomeres 3 and 5. Compared to control embryos (A), the morphants (B, Mo-S1; C, Mo-T) exhibited comparable patterns of pax2.1 and krox20 expression, although the neural plates are substantially wider (compare B′ and C′ with A′). D–F, Similar to what we observe with pax2.1/krox20, the midbrain region, marked by otx2, appears normal in morphant embryos. G–I, Transmitted light images of control (G, Mo-S1mis), and pcdh19 morphant embryos (H, Mo-S1; I, Mo-T). While convergence of the anterior neural plate appears to be impaired in pcdh19 morphants, convergence in the trunk is unaffected. J–L, To confirm that morphogenesis in the trunk is normal, 8–9 somite stage embryos were labeled with riboprobe against myod. PHENOTYPE:
|
|
The neuroepithelium remains intact in pcdh19 morphants. Shown are images of 22 hpf embryos immunostained with antibodies against the apical epithelial marker, ZO-1. (A) Maximum intensity projection of the midbrain–hindbrain region of a wild type embryo (50 sections, spanning 100 μm). ZO-1 labels the apical surface of the neuroepithelium, which lines the midbrain and hindbrain ventricles. (B) In contrast to the ZO-1 labeling in wild type embryos, much of the apical face of the neuroepithelium in morphant embryos is oriented dorsally, more parallel to the surface of the embryo. (C) A YZ-plane through the area indicated in A, highlighting that the apical–basal axis of the neuroepithelium is aligned with the mediolateral axis of the embryo. (D) A YZ-plane through the area indicated in B. In contrast to wild type embryos, much of the apico-basal axis of the neuroepithelium remains aligned with the dorsoventral axis in morphant embryos. PHENOTYPE:
|
|
In vivo timelapse imaging reveals an arrest of convergence movements during neurulation in pcdh19 morphants. (A) Images extracted from a 6 hour timelapse sequence of transgenic embryos expressing a Histone H2A-GFP fusion protein. Each frame is a maximum intensity projection of 20 optical sections (spaced at 5 μm). Shown are three timepoints, at 3 hour intervals, revealing a net migration of cells toward the midline. By 3 h after the initiation of the timelapse, eye primordia have begun to form (red transparency), and the embryo has begun to narrow mediolaterally (blue transparency). Anterior is to the left; scale bar = 50 μm. (B) Images extracted from a 12 hour timelapse of transgenic embryos that have been injected with Mo-S1. In contrast to wild type embryos, cell movement toward the midline is arrested. (C) A kymograph from the 6 hour timelapse image sequence in A, showing the cell convergence. The increase in fluorescence intensity reflects an increase in cell density. The kymograph shows the intensity values measured along a line (11 pixel width, shown as a transparent purple bar in A) at each timepoint. (D) A kymograph from the timelapse image sequence in B, showing a failure of cell convergence. This data was assembled from a 12 hour timelapse sequence. Movement of cells toward the midline is disrupted in pcdh19 morphants, resulting in a lateral accumulation of cells. Compare red arrows in C and D. PHENOTYPE:
|
|
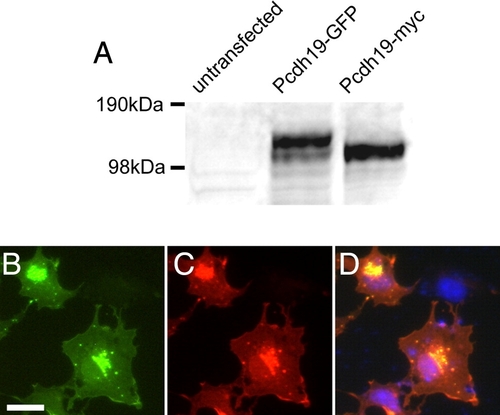
Specificity of anti-Pcdh19 antibody. A, Shown is a Western blot of cell extracts from COS cells that were untransfected or transfected with Pcdh19-GFP or Pcdh19-myc. In untransfected cells, no band is present, while in transfected cells, our antibody detects strong bands corresponding to the sizes of Pcdh19-GFP and Pcdh19-myc, respectively. B–D, Immunocytochemistry of COS cells transfected with zebrafish Pcdh19-GFP. B, Pcdh19-GFP is present in a perinuclear compartment and diffusely along the plasma membrane, as well as in discrete, punctate structures. C, Antibody labeling shows an identical distribution to the Pcdh19-GFP. D, An overlay shows excellent co-localization of Pcdh19-GFP (green) and anti-Pcdh19 (red), while there is no immunolabeling of untransfected cells in the same field (DAPI, blue). |
|
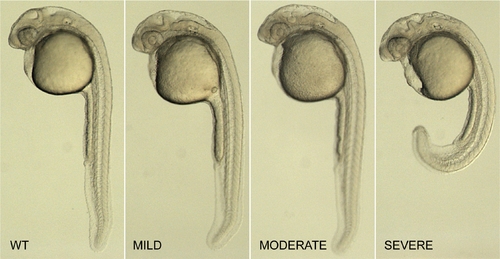
Classification of pcdh19 morphants. Shown are 1 day old zebrafish embryos that are uninjected (WT), or that show increasing severity of morphant phenotype (MILD, MODERATE, SEVERE). Mild embryos are those that exhibit little or no abnormalities of the trunk or the eyes, have a roughly normal-sized brain, but exhibit minor irregularities in the midbrain–hindbrain region. Moderate embryos tend to have slightly smaller eyes, shorter brains along the antero-posterior axis and have a much more disorganized midbrain–hindbrain area. Severe morphants have curved or shortened trunks, severely disorganized brains, ectopic cell masses in the ventricles and, occasionally, areas of pyknotic nuclei. These classes were identical for each of the morpholinos tested (Mo-T, Mo-S1 and Mo-S2), and served as a reliable basis for scoring morpholino injections. |
|
Impaired convergence in the anterior neural plate. Shown are embryos labeled with riboprobes against pax2.1 and krox20. At 2 somite stage, the neural plates of wild type and morphant embryos are comparable in width. However, by 5 somite stage, the neural keel has narrowed considerably in wild type embryos, but remains much wider in pcdh19 morphants. This indicates that morphogenesis occurring prior to the 2 somite stage is largely unperturbed, and that convergence movements occurring between the 2 and 5 somite stages are impaired by the loss of Pcdh19 function. |
Reprinted from Developmental Biology, 334(1), Emond, M.R., Biswas, S., and Jontes, J.D., Protocadherin-19 is essential for early steps in brain morphogenesis, 72-83, Copyright (2009) with permission from Elsevier. Full text @ Dev. Biol.