- Title
-
Fgf and Sdf-1 pathways interact during zebrafish fin regeneration
- Authors
- Bouzaffour, M., Dufourcq, P., Lecaudey, V., Haas, P., and Vriz, S.
- Source
- Full text @ PLoS One
|
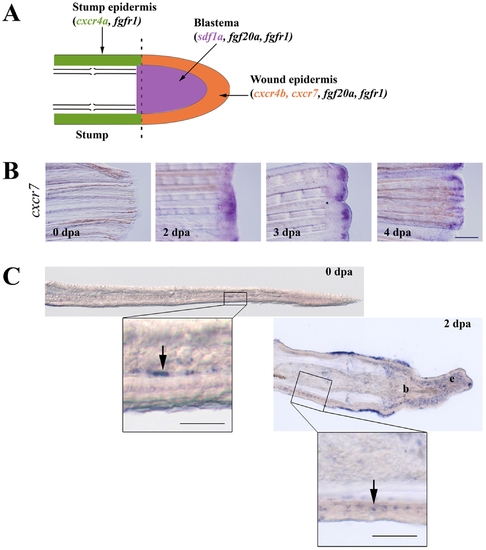
Expression of sdf1 and its receptors during fin regeneration. A: schematic representation of FGF and SDF pathways in caudal fin regeneration. In green: stump epidermis expressing cxcr4a and fgfr1. In red: wound epidermis expressing cxcr4b, cxcr7, fgf20a and fgfr1. In blue: blastema expressing sdf1a, fgf20a and fgfr1. Longitudinal cross section through the dermal ray of a regenerating fin at 2 dpa. The dotted line indicates the amputation plane. B: cxcr7 kinetics of expression during caudal fin regeneration. cxcr7 mRNA expression pattern was analyzed by in situ hybridization on control fin (0 dpa) and in amputated fins allowed to regenerate for 2, 3 and 4 dpa. Scale bar, 100 μm. C: In situ hybridization for cxcr7 on cryosections. cxcr7 mRNA expression pattern was analyzed by in situ hybridization on cryosections of uncut fin (0 dpa) and in amputated fins allowed to regenerate for 2 dpa. Two days post amputation cxcr7 mRNA is detected in the wound epidermis as well as in a few dispersed cells in the stump epidermis (enlarged view). Before amputation, only few mesenchymal cells show a staining for cxcr7. Scale bars, 50 μm. EXPRESSION / LABELING:
|
|
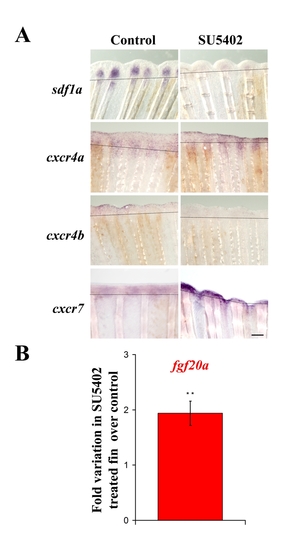
FgfR inhibition modifies sdf1a, cxcr4a, cxcr4b and cxcr7 expression in ongoing fin regenerates. Sections of 2 dpa caudal fins from fish treated with DMSO (control) or FGFR inhibitor (SU5402) after in situ hybridization for sdf1a, cxcr4a, cxcr4b and cxcr7. Scale bar, 100 μm. EXPRESSION / LABELING:
|
|
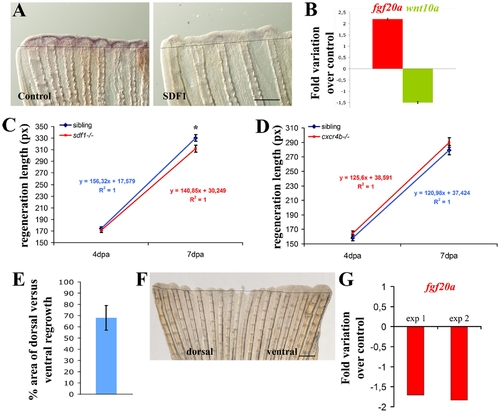
SDF1 inhibits fgf20a and activates Wnt10a expression during fin regeneration. A: Over-expression of SDF1 at the time of amputation turns off fgf20a expression. The protein SDF1, or BSA as a control, were injected into the fin at the time of amputation. Fins were allowed to regenerate for 48 hours before fgf20a expression was checked by ISH. Dotted lines demarcate amputation plane. Scale bar, 100 μm. B: fgf20a expression is enhanced in medusa mutant. fgf20a as well as wnt10a expression were analyzed by quantitative RT-PCR in sdf1a/medusa mutant and sibling fins at 48 hpa. fgf20a expression is increased 2.2±0.03 fold in sdf1 mutant compared to siblings, while wnt10a expression is reduced 1.5±0.04 fold. Average values (±s.e.m.) from experiments performed in triplicate are presented. C, D: Fins regenerate slower in sdf1/medusa mutant compared to siblings (C) whereas cxcr4b mutant fish regenerates caudal fin with no abnormality (D). Length of the third dorsal and ventral regenerating fin ray of the regenerates were measured in ody-/- mutant fish and siblings (ody+/- and ody+/+) (D) and in medusa/sdf1-/- mutant fish and siblings (sdf1+/+ and sdf1+/-) (C) at 4 and 7 dpa. Precise measures were made in pixels (1 pixel corresponding to 3 μm). The average length of the regenerate allows to calculate the regeneration speed. No significant difference was observed between ody-/- mutant fish and siblings. Two independent experiments were performed. Experiment 1: n = 5 ody-/-, n = 7 siblings. Experiment 2 n = 5 ody-/-, n = 7 siblings (data not shown). Errors bars represent the s.e.m. of the average regenerate length. Fins regenerate slower in sdf1/medusa mutant n = 18 siblings (sdf1+/+ and sdf1+/-), n = 18 medusa/sdf1-/-. Errors bars represent the s.e.m. of the average regenerate length (* p<0.05). E–F: cxcr7 overexpression inhibits blastema formation. Plasmid DNA expressing cxcr7 (pCS2-cxcr7) was injected into the dorsal half fin whereas an empty plasmid (pCS2) was injected into the ventral part of the fin at the time of amputation prior to electroporation. Fins were allowed to regenerate for 48 hours before scoring blastema formation (n = 8). The percent area of dorsal versus ventral regrowth is presented in E and a representative fin in F. Scale bar, 500 μm. Dotted lines demarcate amputation plane. G: cxcr7 overexpression inhibits fgf20 expression. Plasmid DNA expressing cxcr7 (pCS2-cxcr7) or an empty plasmid (pCS2) as control were electroporated into total fin at the time of amputation. Fins were allowed to regenerate for 48 hours before checking fgf20 expression by quantitative PCR. Two independent experiments are presented in G. Experiment 1: n = 5; experiment 2: n = 5. |
|
FgfR inhibition modifies, cxcr4a, expression in ongoing fin regenerates. Sections of 2 dpa caudal fins from fish treated with DMSO (control) or FGFR inhibitor (SU5402) after in situ hybridization for, cxcr4a. Scale bar, 100 μm. |
|
A. SU5402 treatment modifies sdf1, cxcr4a, cxcr4b and cxcr7 expression. mRNA expression was analyzed by in situ hybridization on 2 dpa caudal fins from fish treated with DMSO (control) or FGFR inhibitor (SU5402). Scale bar, 100 μm. B. fgf20a expression is enhanced in SU5402 treated fins : fgf20a expression was analyzed at 48 hpa by quantitative RT-PCR in SU540-treated fish and DMSO fins as control. fgf20a expression is increased 1,9-fold when the FGF pathway is blocked compared to control. Average values (±s.e.m.) from experiments performed in quadruplicate are presented (** p<0.01). |
|
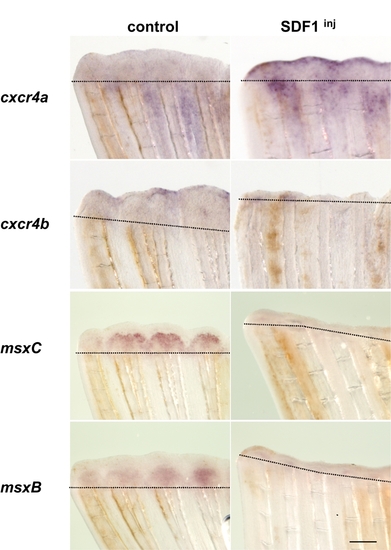
SDF1 overexpression inhibits Fgf downstream genes expression The SDF1 protein, or BSA as a control, were injected in the fin at the time of amputation. Fins were allowed to regenerate for 48 hours before being stained for Fgf20 downstream genes cxcr4a, cxcr4b, msxb and msxc expression. Dotted lines demarcate amputation plane. Scale bar, 100 μm. |
|
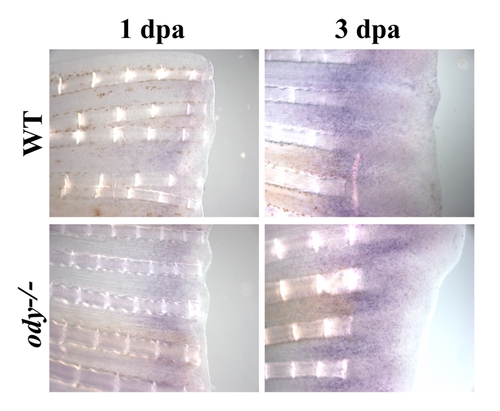
cxcr4a mRNA expression in odysseus fins (ody-/-) regenerating fins cxcr4a mRNA expression pattern was analyzed by in situ hybridization on control fin (wt) and on odysseus fins (ody-/-) after 1 or 3 dpa. No difference in expression of cxcr4 was observed between odysseus fins (ody-/-) and wild type fish. |