Fig. 1
- ID
- ZDB-FIG-250822-56
- Publication
- Cakan-Akdogan et al., 2025 - Novel anti-VEGF scFv antibodies with superior in vitro and in vivo activities
- Other Figures
- All Figure Page
- Back to All Figure Page
|
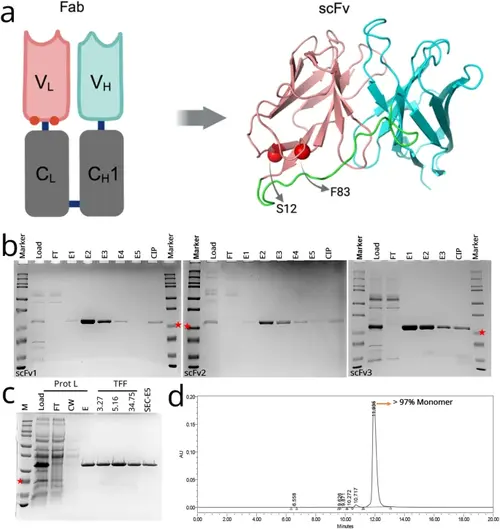
Structure, production, and purification of anti-VEGF scFv variants. (A) Graphic representing the location of mutated residues (red spheres) in the original ranibizumab template, and the modelled structure of the scFv variant. Positions for selected mutations of F83E and S12M are represented by red spheres. Variable heavy chain (VH), light chain (VL) and linker are colored cyan, salmon and green, respectively. (B) Protein L-purified proteins were analyzed on SDS-PAGE gel for scFv1 (left), scFv2 (middle), scFv3 (right). FT: flow-through, E1-E5: Elution fractions, CIP: Clean-in-place (proteins stuck onto the Protein L column). scFv bands at around 25 kDa (indicated with red stars). (C) Samples from Protein L purification and protein concentration steps are analyzed on SDS-PAGE. Prot L: Protein L Chromatography, TFF: Tangential Flow Filtration, FT: Flow-Through, CW: Column Wash, E: Elution, SEC: Size Exclusion Chromatography. (D) Representative SEC-HPLC chromatogram of the purified scFv1. Detection at 280 nm absorbance (mAU) and conductivity (mS/cm) was plotted. Monomer peak was observed at 12 min. |