Fig. 3
- ID
- ZDB-FIG-240419-22
- Publication
- Ambrosio et al., 2024 - LiverZap: a chemoptogenetic tool for global and locally restricted hepatocyte ablation to study cellular behaviours in liver regeneration
- Other Figures
- All Figure Page
- Back to All Figure Page
|
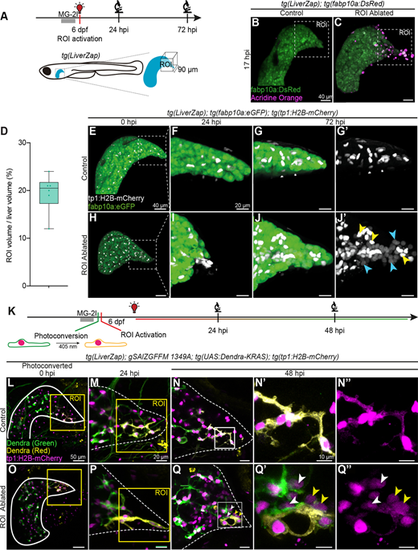
LiverZap ROI activation triggers a local LPC response. (A) Local activation of LiverZap using a confocal microscope WLL tuned at 660 nm. (B,C) Acridine Orange (magenta) marks apoptotic cells after ROI LiverZap activation; hepatocytes express tg(fabbp10a:eGFP) (green; N=3, n=3). Maximum intensity projections show segmented livers. (D) Quantification of ROI ablation volume by normalisation to whole liver volume at 0 hpi (N=3, n=6). Box limits represent the interquartile range, horizontal line the median and whiskers the minimum and maximum data points. (E-J′) tg(fabp10a:eGFP)-positive hepatocytes (green) and tg(tp1:H2B–mCherry)-labelled BECs (grey) were visualised after ROI LiverZap activation (N=3, n=6). At 72 hpi, LPC-derived hepatocytes are observed in ROI-ablated livers expressing low mCherry (J′, blue arrowheads), whereas BECs have high mCherry (J′, yellow arrowheads). Because Notch is active in the adjacent intestine, a manual mask was generated to highlight the tg(tp1:H2B-mCherry) signal in the liver. See Fig. S3A,C for unmasked view of the same sample. Masked maximum intensity projections are shown. Dotted squares in E and H depict the ROIs shown in F-G′ and I-J′. (K) Dendra in BECs was photoconverted prior to LiverZap-mediated ROI ablation. Consecutive imaging monitored all specimens live at 24 and up to 72 hpi (N=4, n>5). (L-Q″) Dendra expression (green) in BECs was photoconverted prior to ROI LiverZap activation (yellow); BECs express tg(tp1:H2B–mCherry) in controls (N=3, n>4). At 48 hpi, the ROI contains BEC-derived hepatocytes (mCherrylow) showing converted (yellow arrowheads) or non-converted (white arrowheads) Dendra. Maximum intensity projections are shown in L,M,O,P; 5 µm projections are shown in N-N″,Q-Q″. Scale bars: 40 µm (B,C,E,H); 20 µm (F-G′,I-J′,M-N″,P-Q″); 50 µm (L,O). |