|
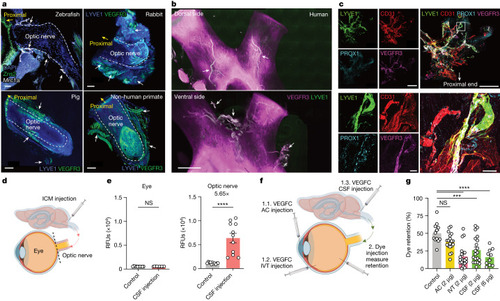
Optic nerve sheath lymphatics drain the posterior eye. a,b, Immunostaining of sections of optic nerve from zebrafish with lymphatics labelled for Mrc1a (white arrows; a, top left) and iDISCO immunolabelling of meningeal lymphatic vessels of rabbit (a, top right), pig (a, bottom left), non-human primate (a, bottom right) and human (b) optic nerve and chiasma, with lymphatics showing colocalization of LYVE1 and VEGFR3 (white arrows). DAPI, 4′,6-diamidino-2-phenylindole. c, Whole-mount wild-type mouse optic nerve sheaths stained for LYVE1, CD31, PROX1 and VEGFR3. The images at the bottom show a higher-magnification view of the area highlighted in the merged image at the top right. Scale bars, 50 μm (a, top left; c, bottom merged), 500 μm (a, top right; c, top merged), 1,000 μm (a, bottom left and right) and 3,000 μm (b). d, Schematic of ICM injection and the eye and optic nerve dissection for e. e, Dye was ICM injected, and fluorescence signal intensity was measured 1 h later in the eye and optic nerve (control, n = 11; CSF injection, n = 11). ****P < 0.0001. f, Schematic of injection methods for g. g, VEGFC was injected through the AC, IVT or ICM administration route. Two days later, dye was IVT injected into the eye, and the percentage of dye retention was measured 12 h after dye injection (control, n = 12; AC, n = 19; IVT, n = 20; CSF (2 μg), n = 20; CSF (6 μg), n = 10) ****P < 0.0001; ***P < 0.0002. Data are shown as mean ± s.e.m. P values were calculated using a one-way ANOVA with multiple comparisons testing (Dunnett) or two-tailed unpaired Student’s t-test. The graphics in d,f were created with BioRender.com. Source Data
|