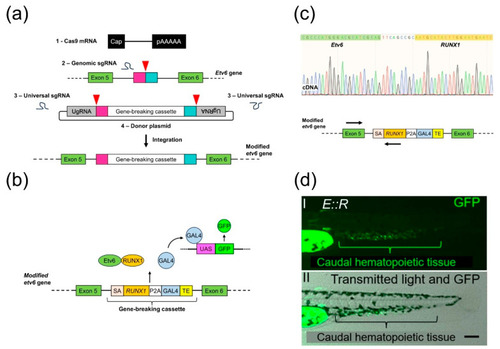
Generation of the ETV6::RUNX1 (E::R) knock-in line. (a) A schematic illustrating the integration of the gene-breaking cassette into intron 5 of the etv6 gene using the GeneWeld technique. The process required four reagents: (1) Cas9 mRNA, (2) genomic sgRNAs, (3) universal sgRNAs, and (4) a donor plasmid containing the gene-breaking cassette flanked by 48 bp homology arms and by universal sgRNA target sites. In vivo, Cas9-induced a targeted double-strand break (DSB) in the genome and generated two targeted DSBs in the donor plasmid, liberating the cassette along with the short homology arms. Subsequently, integration of the cassette followed. Red arrowheads: Cas9 nuclease-induced DSBs; pink and turquoise rectangles: homology arms; UgRNA: universal sgRNA target sites. (b) Schematic of the E::R zebrafish model showing the integrated gene-breaking cassette and the UAS:GFP transgene. E::R fusion protein and GAL4-VP16 protein expression are driven by the endogenous etv6 promoter. GAL4-VP16 binds to UAS enhancer sequences, leading to GFP expression. SA: splice acceptor; TE: ocean pout antifreeze gene transcriptional termination and polyadenylation sequence. (c) Sequence of the in-frame splicing of the etv6 and RUNX1 transcripts. Primers used in the RT-PCR are either complementary to a region in exon 5 of etv6 or the inserted human RUNX1 cDNA, as indicated by arrows on the schematic of the etv6 locus (Table S5). (d) Lateral view of a 2 day old E::R zebrafish larva with two images: (d(I)) GFP fluorescence in the caudal hematopoietic tissue and (d(II)) a merged image of GFP fluorescence with transmitted light, providing the structural context. Scale bar: 50 µm.
|

