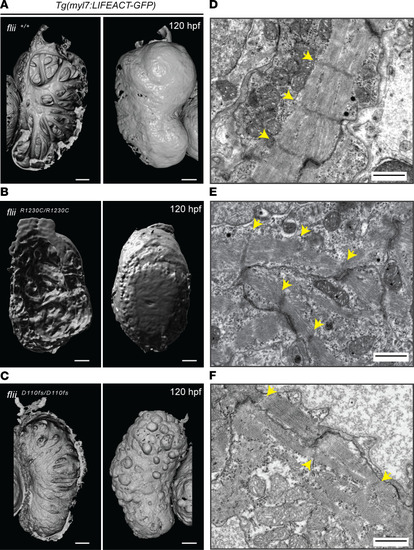
Figure 3
- ID
- ZDB-FIG-231003-11
- Publication
- Ruijmbeek et al., 2023 - Bi-allelic variants in FLII cause pediatric cardiomyopathy by disrupting cardiomyocyte cell adhesion and myofibril organization
- Other Figures
- All Figure Page
- Back to All Figure Page
|
Flii dysfunction results in myofibrillar architectural abnormalities of the ventricular myocardium. ( |