Figure 6—figure supplement 2.
- ID
- ZDB-FIG-191230-88
- Publication
- Harrison et al., 2019 - Late developing cardiac lymphatic vasculature supports adult zebrafish heart function and regeneration
- Other Figures
-
- Figure 1
- Figure 1—figure supplement 1.
- Figure 2—figure supplement 1.
- Figure 2.
- Figure 3
- Figure 3—figure supplement 1.
- Figure 3—figure supplement 2.
- Figure 4
- Figure 4—figure supplement 1.
- Figure 5
- Figure 5—figure supplement 1.
- Figure 6
- Figure 6—figure supplement 1.
- Figure 6—figure supplement 2.
- Figure 6—figure supplement 3
- Figure 6—figure supplement 4.
- Figure 6—figure supplement 5.
- All Figure Page
- Back to All Figure Page
|
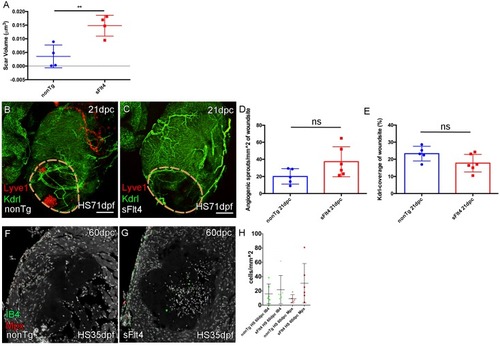
(A) The volume of scar in hearts lacking ventricle cardiac lymphatic vessels (after hsp70l:sflt4 induction from 1mpf; no induction post injury) is significantly larger at 60dpc (n = 4; **p=0.0075; one outlier with abnormally large scar, 0.036 μm3 and 0.051 μm3, removed from nonTg and sFlt4 groups respectively). (B–C) Whole-mount confocal imaging of 21dpc adult transgenic zebrafish hearts (heat shocked from 71dpf to 230dpf) expressing arterially and endocardially enriched endothelial marker kdrl:GFP (green: B, C) and lymphatic endothelial marker lyve1:RFP (red: B, C). Pre-injury induction of hsp70l:sflt4 (from 71dpf) does not inhibit angiogenesis into the woundsite after injury. (D) Non-significant (ns) slight increase in angiogenic sprouts into the woundsite is observed at 21dpc with juvenile-induced pre-injury hsp70l:sflt4. (E) A reciprocal, but also non-significant, small decrease in average vessel coverage of the wound site is observed in the same zebrafish. (F–H) Some hearts lacking ventricle lymphatic vessels show higher numbers of regeneration site immune cells at 60dpc, but overall there is no significant difference. Scale bars 200 μm. |