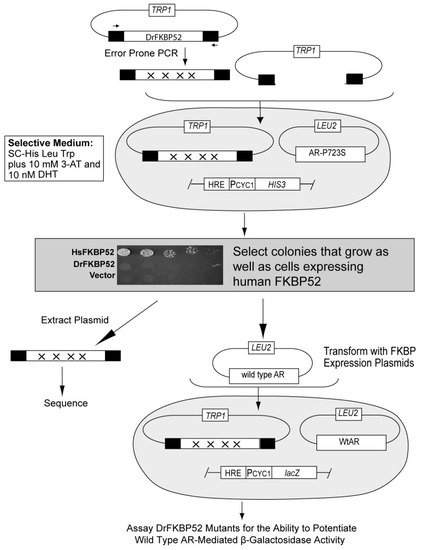
Selection scheme for Danio rerio FKBP52 gain-of-function mutants. Libraries of random DrFKBP52 mutants were independently generated by error-prone PCR using primers (horizontal arrows) binding upstream in the GAPDH promoter (PGPD) or downstream in the transcriptional terminator (Term). Randomly generated mutant constructs were co-transformed with a linearized vector to facilitate homologous recombination between the common promoter and terminator regions on these fragments reconstituting TRP1-marked expression plasmids harboring the DrFKBP52 mutants. The parental strain contains a LEU2-marked androgen receptor (AR)-P723S expression plasmid and integrated HIS3 reporter gene driven by a hormone-responsive promoter element (HRE) such that growth in histidine-lacking medium is dependent on AR-P723S activity. Transformants were plated on selective growth medium supplemented with 10 mM 3-amino-1,2,4-triazole and 10 nM dihydrotestosterone (DHT), and colonies that grew, as well as those expressing human FKBP52, were selected for further analysis. Mutants exhibiting the gain-of-function phenotype were extracted from yeast and co-transformed with wild type AR into a secondary strain containing a hormone-responsive LacZ reporter plasmid, and assayed for the ability to potentiate wild type AR-mediated β-galactosidase activity. DrFKBP52 mutants that gained the ability to potentiate AR activity in these assays were sequenced to identify relevant mutations (inset). Yeast strains containing a hormone-inducible HIS3 gene and expressing AR-P723S plus either Vector, HsFKBP52, or DrFKBP52 were serially diluted and spotted on selective medium containing a growth-limiting concentration of 10 nM DHT (inset).
|

