Fig. 8
|
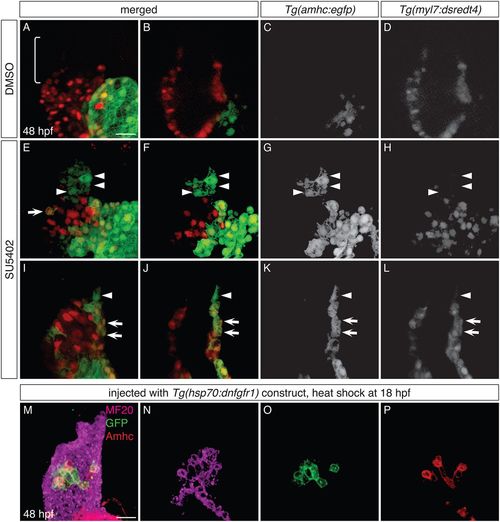
FGF signaling influences the identity of both early-differentiating and late-differentiating ventricular cardiomyocytes. (A-L) Three-dimensional reconstructions (A,E,I) and single optical sections (B-D,F-H,J-L) of live Tg(amhc:egfp);Tg(myl7:dsredt4) embryos; lateral views at 48 hpf, after exposure to DMSO (A-D) or SU5402 (E-L) from 18 hpf. (A-D) As expected, control embryos display eGFP in the atrium and not in the ventricle, and the arterial pole (bracket) is composed of late-differentiating cardiomyocytes (DsRed−) (n=5). (E-L) In SU5402-treated embryos, eGFP is routinely found in the ventricle (n=10). Two representative embryos (E-H and I-L) illustrate detection of eGFP both in early-differentiating cardiomyocytes (arrows, eGFP+DsRed+; n=10/10) and in late-differentiating cardiomyocytes (arrowheads, eGFP+DsRed−; n=7/10). (M-P) Immunofluorescence for MF20 (magenta), GFP (green) and Amhc (red) at 48 hpf indicates mosaic expression of dnfgfr1-egfp in the ventricle of an embryo that was injected with the Tg(hsp70:dnfgfr1) construct and then heat shocked at 18 hpf; three-dimensional reconstruction (M) and single optical sections (N-P). The dnfgfr1-egfp-expressing cells in the proximal ventricle exhibit variegated levels of Amhc (P; n=3). Scale bars: 30 μm. |