Fig. S1
- ID
- ZDB-FIG-170220-16
- Publication
- Linsley et al., 2017 - Congenital myopathy results from misregulation of a muscle Ca2+ channel by mutant Stac3
- Other Figures
- All Figure Page
- Back to All Figure Page
|
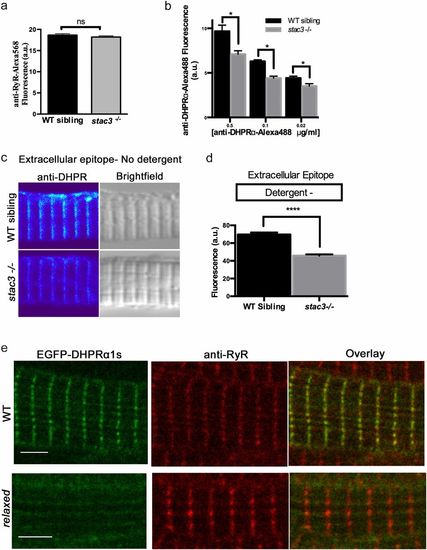
Loss of Stac3 does not prevent trafficking of DHPRα to triads. (A) Quantification of the mean of immunofluorescence labeling (±SEM) of Alexa 568 directly coupled to anti-RyR in stac3−/− at triads compared with WT siblings (n = 60, t test, P = 0.29). (B) Quantification of the mean of immunofluorescence labeling of Alexa 488 directly coupled to anti-DHPRα1S at different concentrations in stac3−/− at triads compared with WT siblings (n = 40 WT sibling, n = 40 stac3−/−, t test, *P < 0.0001). (C) Immunofluorescence (Left) and bright-field images (Right) of WT sibling and stac3−/− disassociated myotubes labeled without detergent with anti-DHPRα1S that recognizes an extracellular epitope. (D) Histogram showing that there is a decrease in T-tubule triadic DHPRα1S in stac3−/− dissociated myotubes (n = 160 WT sibling, n = 125 stac3−/−, t test, ****P < 0.0001). (E) Anti-Ryr immunolabeling of a fixed muscle fiber expressing EGFP-DHPRα1S showing that EGFP-DHPRα1S does not localize to triads in a relaxed mutant fiber, whereas it does in a WT fiber. SEMs are indicated. n.s., not significant. (Scale bars, 4 μm.) |