Fig. 7
|
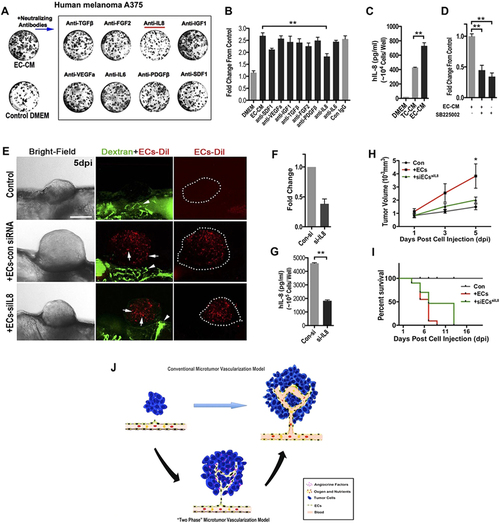
ECs-derived IL-8 mediated the initial growth of A375 micro-xenografts in vitro and in vivo. (A) Representative images of human A375 melanoma clones cultured in phEC-CM supplemented with different neutralizing antibodies for 4 days. (B) Quantitative analysis of the A375 tumor cells in phEC-CM with different neutralizing antibodies. (n = 5 wells for each group). (C) Quantitative analysis of the A375 tumor cells in phEC-CM treated with SB225002 (1 µM, inhibitor of IL-8 receptor CXCR2) inhibitor (n = 5 wells for each group). (D) hIL-8 concentration of DMEM, TC-CM and EC-CM (n = 3 wells for each group). (E) Representative images of A375 xenografts with or without incorparated ECs (Arrows), primary ECs (HUVECs) were pre-stained by CM-Dil, blood flow (Arrowheads) in zebrafish were imaged by FITC-dextran (2 million MW). Dotted lines indicate the location of microtumors. (F,G) Evaluation of IL-8 expression by q-PCR and Elisa at 24 h after ECs was treated by siRNA. (H,I) Quantitative analysis of xenografts growth rate and the survive rate of zebrafish hosts after implanted with A375 tumor cells alone or hECs: A375 cells mixture (1:10). (n > 20 fish for each group). (J) The “two-phase” model of ‘angiogenic switch’. Angiogenic neovessels are conventionally believed infiltrating into the avascular microtumor as circulatory sprouts with blood perfusion. We propose that initial endothelial cords in microtumors remain non-circulatory and drive tumor growth through a paracrine mechanism by releasing endothelium-derived proliferative factors (angiocrine factors), before they support tumor progression by supplying oxygen and nutrients through the blood circulation. |