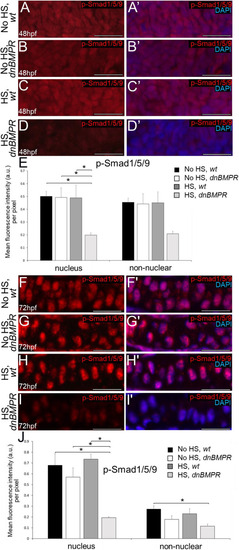
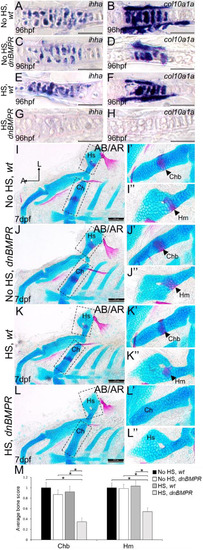
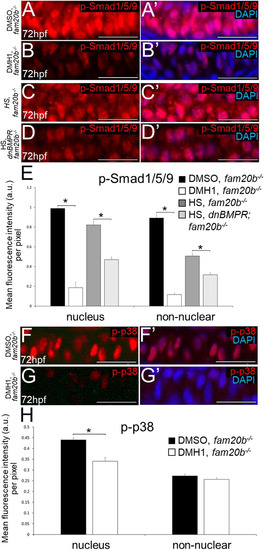
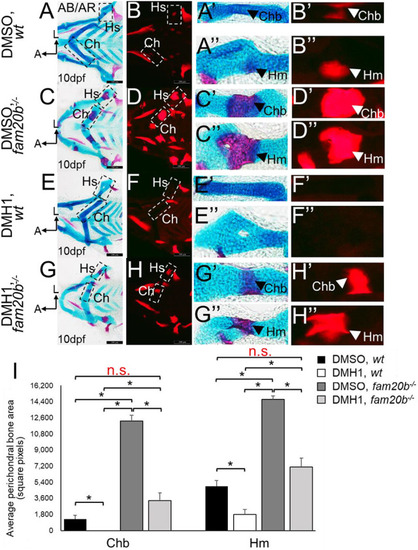
Inhibition of BMP signalling by DMH1 in wild types reduces cartilage maturation gene expression and perichondral bone formation. (A-D′) Immunostaining of wild-type chondrocytes at 72 hpf (i.e. after 24 h of treatment) demonstrated that DMH1 treatment reduced p-Smad1/5/9 (B,B′) and p-p38 (D,D′) immunoreactivity, compared with DMSO-treated control chondrocytes (A,A′,C,C′). (E) Quantitative image analyses (n=3 for each group) revealed significant decreases in p-Smad1/5/9 and p-p38 levels in nuclei, as well as a significant decrease in p-Smad1/5/9 in non-nuclear regions, of DMH1-treated chondrocytes, compared with DMSO-treated control chondrocytes (*P<0.05, one-way ANOVA and paired Student's t-test). (F-I) Compared with DMSO-treated control chondrocytes (F,G), DMH1 treatment decreased expression of the chondrocyte maturation genes ihha and col10a1a at 4 dpf (i.e. after 2 days of treatment; H,I). These are representative images from at least 12 samples for each group (at least six samples each from two clutches). (J-K″) Compared with DMSO-treated controls (J-J″), DMH1 treatment appeared to decrease perichondral bone at 7 dpf (i.e. 3 days after treatment ended; K-K″). (L) Quantitation of 100 larvae (20 larvae each from five clutches) for each experimental group confirmed a significant decrease in perichondral bone in DMH-treated wild types (*P<0.05, one-way ANOVA and paired Student's t-test). Scale bars: 50 μm (A-D′,F-I); 200 μm (J,K). A, anterior; AB/AR, Alcian Blue/Alizarin Red; a.u., arbitrary units; Bsr, branchiostegal ray; Ch, ceratohyal cartilage; Chb, ceratohyal bone; Hm, hyomandibular bone; Hs, hyosymplectic cartilage; L, lateral; Op, opercle; wt, wild type.
|