- Title
-
The Effects of Aging on Rod Bipolar Cell Ribbon Synapses
- Authors
- Shrestha, A.P., Rameshkumar, N., Boff, J.M., Rajmanna, R., Chandrasegaran, T., Frederick, C.E., Zenisek, D., Vaithianathan, T.
- Source
- Full text @ Cells
|
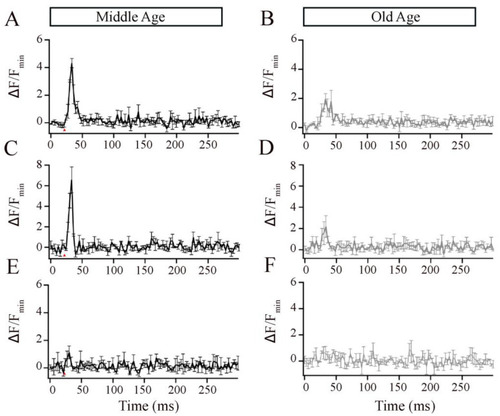
Retinal bipolar cells isolated from old-aged zebrafish exhibited no changes in their brief release properties relative to middle-aged fish. ( |
|
The retinal bipolar cells isolated from older-aged zebrafish exhibit changes in the numbers and length of their synaptic ribbons. ( |
|
The morphology of bipolar cell ribbon synapses in the retinal IPL was altered in older-aged zebrafish. ( |
|
The ribbon synapses in the Mb1 bipolar cells from older-aged zebrafish exhibited altered Ca2+ responses after brief depolarization. ( |
|
The local Ca2+ responses elicited by brief depolarization at an Mb1 bipolar cell synaptic ribbon were altered in older-aged zebrafish. ( |