- Title
-
Zebrafish as a high throughput model for organ preservation and transplantation research
- Authors
- Da Silveira Cavalcante, L., Lopera Higuita, M., González-Rosa, J.M., Marques, B., To, S., Pendexter, C.A., Cronin, S.E.J., Gopinathan, K., de Vries, R.J., Ellett, F., Uygun, K., Langenau, D.M., Toner, M., Tessier, S.N.
- Source
- Full text @ FASEB J.
|
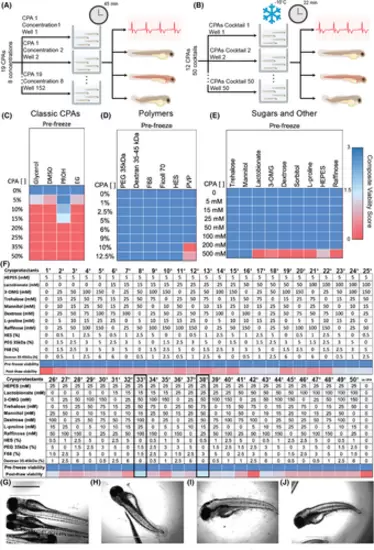
Individual CPA toxicity assay (A) and identification of best performing external CPAs cocktail capable of retaining larvae viability after 22 min of storage at −10°C when immersed in CPAs (B). Larvae viability was determined by quantifying heart rate, dehydration, blood circulation, and fish morphology and reported as the External Composite Viability Score (CVSExt). (C–E) Four classic CPAs, six polymers, seven sugars, and two other components (total of 19) were tested to determine their individual toxicity prefreeze at eight different concentrations by incubating larvae for 45 min (n = 4 animals per CPAs concentration). (F) Twelve CPAs, from the previously tested nineteen, were arranged in a L50 Taguchi experimental design and tested at different concentrations (7) yielding 50 CPAs cocktails (n = 4 animals per CPAs cocktail). Prefreeze toxicity was determined after 30 min of incubation, with CPAs cocktails 33+ and 38+ resulting in the best larvae outcomes as determined by above threshold (>1.5) post-thaw CVSExt (1.75 and 1.625 respectively). Storage with above-threshold CPAs cocktails resulted in post-thaw retention of larva morphology (G) compared to untreated or unfrozen larvae (H), whereas storage without CPAs (I) or below the-threshold CPAs (J) resulted in significant loss of morphology and overall embryo integrity. Scale bar 1000 um. |
|
Identification of best performing internal CPAs cocktail capable of retaining larvae (Tg(cmlc2:nGFP, mKate-CAAX)fb18Tg) viability after 22 min of storage at −10°C when injected through the duct of Cuvier and immersed in CPAs cocktail 33+ |
|
CPAs cocktails 2, 6, 7, and 8 partially retain cardiac function of adult zebrafish hearts after 25 min of storage at −10°C when loaded via immersion (n = 6 animals per treatment). (A) Representative microscopic images of adult zebrafish hearts treated with different CPAs cocktails. (B) Heart rate (beats per minute—bpm) was best retained in hearts treated with CPAs 2, 6, 7, and 8 with no statistical difference seen between fresh and the time control hearts. (C) Cardiac metabolism was best retained by CPAs 6 having no statistical difference with Fresh controls. All other CPAs cocktails resulted in statistically lower cell metabolism when compared to Fresh controls and similar metabolism of hearts frozen without CPAs. All statistical data is expressed as mean ± standard deviation and analyzed via ordinary one-way ANOVA with Tukey's multiple comparison test. *p < .05, **p < .01, ***p < .001, ****p < .0001. |
|
CPAs cocktail 6 partially retained cardiac function of adult zebrafish hearts after 24, 72, and 120 h of storage at −10°C when loaded via perfusion (n = 6 animals per treatment). (A) Heart beat analysis for hearts stored in CPAs 6 resulted in similar heart rate recovery at all storage times. No statistical difference was seen between heart rate of CPAs cocktail 6 hearts over time or when compared to the beat ratio of hearts stored in clinical standard conditions (HypothermicCON) at each individual storage times. (B) Similarly, storage in CPAs cocktail 6 resulted in ~40% of cardiac tissue to remain viable post-thaw, irrespective of freezing time, suggesting temperature-dependent injury but no time-dependent injury. (C) Separate analysis of ventricular and atrial beat for hearts stored in CPA 6 resulted in similar atrial and ventricular contraction (contraction per minute—cpm) recovery at all storage times, with the atrium contracting over twice as often as the ventricle. No statistical difference was seen between beat ratio of CPAs cocktail 6 hearts over time or when compared to the beat ratio of hearts stored in clinical standard conditions (HypothermicCON) at each individual storage times (D) Representative histological images of TUNEL and DAPI staining. Scale bar 250 μm. All statistical data are expressed as mean ± standard deviation. Heart rate and Cardiac Viability were analyzed via a two-way ANOVA and Tukey–Kramer HSD post-hoc analyses. Contraction rate was analyzed via an ordinary one-way ANOVA with Tukey's multiple comparison test. ****p < .000. |