- Title
-
Light-sheet autofluorescence lifetime imaging with a single-photon avalanche diode array
- Authors
- Samimi, K., Desa, D.E., Lin, W., Weiss, K., Li, J., Huisken, J., Miskolci, V., Huttenlocher, A., Chacko, J.V., Velten, A., Rogers, J.D., Eliceiri, K.W., Skala, M.C.
- Source
- Full text @ J. Biomed. Opt.
|
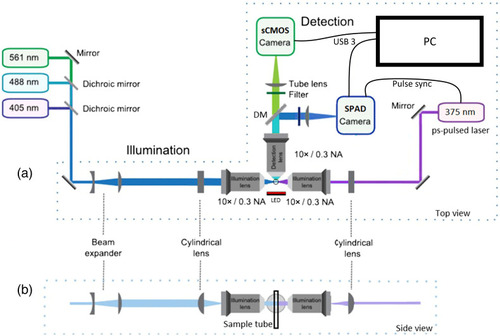
Schematic of the light-sheet FLIM microscope (adapted with permission from Ref. |
|
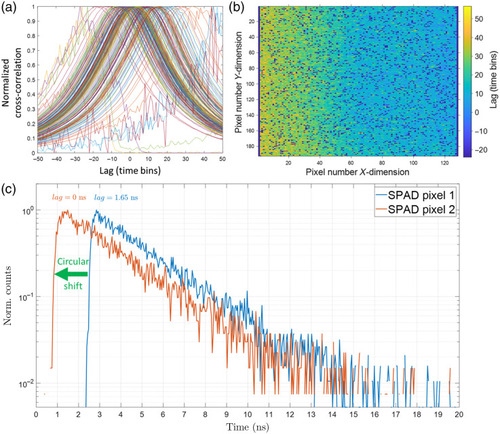
IRF. To measure the combined IRF of the excitation laser (Omicron QuixX 375-70PS) and the SPAD camera (Horiba FLIMera), a fraction of the excitation laser pulses (50 MHz pulse repetition rate) was directly imaged onto the FLIMera camera by imaging a retroreflector target in an epi-illuminated widefield microscope setup. The 20-ns time axis consists of 486 time bins of 41.1 ps width. The IRF has an FWHM of 380 ps. The DCR of individual pixels can be measured from the non-zero tail of the pixels’ IRF. |
|
FLIMera SPAD array camera inter-pixel delay map. To compensate for the inter-pixel timing skew and synchronize the rise time of all |
|
Fluorescence lifetime fitting of coumarin 6 in ethanol. A saturated solution of coumarin 6 in ethanol was loaded into an FEP sample tube and imaged on the light-sheet SPAD system with 0.4 mW excitation laser power and 3 s integration time using a green emission filter ( |
|
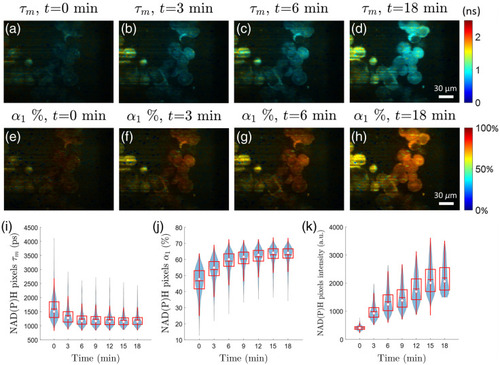
Cyanide treatment of PANC-1 cells confirms the sensitivity of the light-sheet FLIM system to NAD(P)H fluorescence lifetime changes ( |
|
Light-sheet imaging of mCherry intensity and NAD(P)H lifetimes in live neutrophils |