- Title
-
Embryonic organizer formation disorder leads to multiorgan dysplasia in Down syndrome
- Authors
- Liu, Y., Lin, Z., Peng, Y., Jiang, Y., Zhang, X., Zhu, H., Zhang, L., Chen, J., Shu, X., Luo, M., Xie, D., Chen, Y., Liao, H., Liu, M., Zhang, X., Liu, S., Wang, H., Zhou, B., Sun, H.
- Source
- Full text @ Cell Death Dis.
|
a Morphogenesis of live control (injection with GFP mRNA) and DYRK1A-overexpressed (injection with DYRK1A mRNA) embryos. The arrowheads indicate the dorsal side of the embryo. b Fusion of eyes caused by DYRK1A overexpression resembled the phenotype of lefty1 mRNA injection. c Germ layer marker expression detected by WISH during gastrulation. Orientation: ntla, sox17, and otx2, dorsal views with an animal pole to the top; others, animal-pole views with dorsal to the right. The numbers indicated in each picture are the number (left) of affected embryos with a phenotype similar to what is shown in the picture and the total number (right) of observed embryos. The same number labeling was used thereafter. |
|
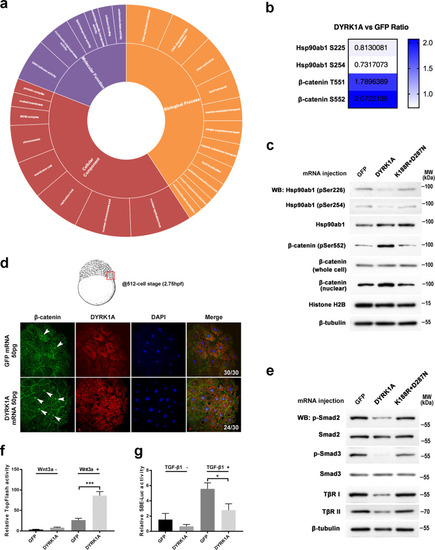
a Gene Ontology (GO)-based enrichment analysis of regulated protein phosphorylation sites on the ontology of biological processes, cellular components, and molecular functions. b The quantified phosphorylation sites of β-catenin and Hsp90ab1 were detected by phosphoproteome analysis in DYRK1A-overexpressed embryos. c Regulation of phosphorylation sites of β-catenin and Hsp90ab1 was verified using western blot assay in zebrafish embryos. d Whole-mount immunostaining of β-catenin at the 512-cell stage showed that nuclear β-catenin increased in the DYRK1A-overexpressed embryo. Whole embryos were flattened and viewed from the animal pole by confocal microscopy. e DS embryo model blocks Smad2/3 phosphorylation detected by western blot. f DYRK1A enhanced the expression of the Wnt reporter TopFlash in HEK-293T cells. (g) DYRK1A decreased TGF-β induced SBE-luc activity in HEK-293T cells. |
|
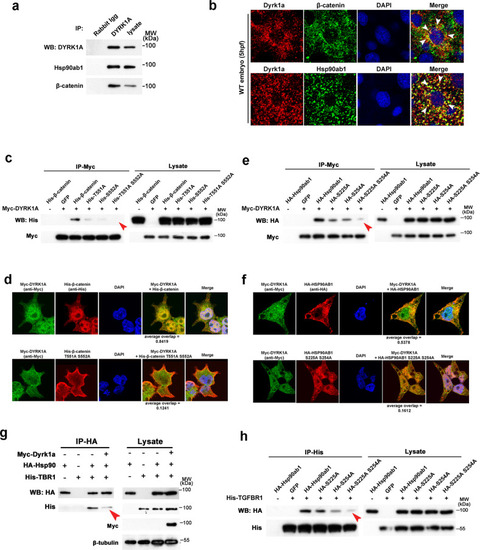
a Co-immunoprecipitation (Co-IP) of endogenous DYRK1A and β-catenin/Hsp90ab1 in zebrafish embryos. b Co-localization of DYRK1A and β-catenin/Hsp90ab1 in the zebrafish embryo. Arrowheads indicate colocalized sites. c Co-IP shows the interaction of DYRK1A with β-catenin is abolished by T551A and S552A mutation. d Immunofluorescence analysis in HEK293 cells shows the reduced localization of β-catenin T551A and S552A mutant with DYRK1A. e Co-IP shows the interaction of DYRK1A with Hsp90ab1 is impaired by S225A and S254A mutation. f Immunofluorescence analysis in HEK293 cells shows that Hsp90ab1 S225A and S254A mutant is less co-localized with DYRK1A. g Co-IP/western blot analysis in HEK293 cells shows that DYRK1A overexpression decreases the protein–protein interaction between Hsp90ab1 and TGF-β receptor. h Hsp90ab1 S225 and S254 sites are required for its interaction with the TGF-β receptor. Red arrowheads in (c, e, g, h) indicate the decreased interaction of proteins. |
|
a Injection of TGF-β legend sqt mRNA and treatment of Wnt-β-catenin inhibitor ICG-001 simultaneously rescue the abnormal phenotype of the DYRK1A-overexpressed embryo. b sqt mRNA and ICG-001 also recover the ectopic expression of organizer marker genes gsc and chd in the DYRK1A-overexpressed embryo. |
|
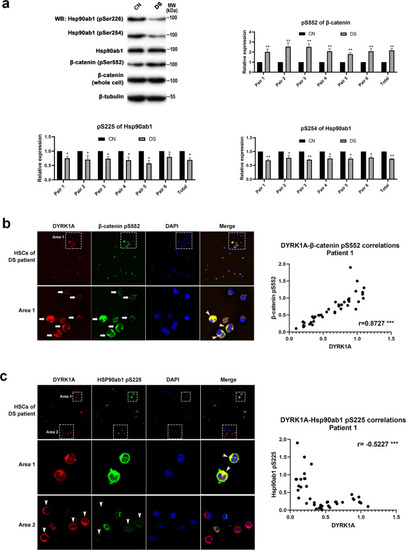
a Representative western blot assay and corresponding statistics to detect the phosphorylation sites of β-catenin and Hsp90ab1 in amniocytes. b DYRK1A expression positively correlated with the phosphorylation level of β-catenin Ser552 site in HSCs from DS patients. Arrows show the detected HSCs, arrowheads show the colocalization of two proteins. c DYRK1A expression negatively correlated with the phosphorylation level of the Hsp90ab1 S225 site in HSCs from DS patients. Arrowheads in Area 1 show the colocalization of two proteins, and arrowheads in Area 2 show the detected HSCs. |