- Title
-
Heterogeneities of zebrafish vasculature development studied by a high throughput light-sheet flow imaging system
- Authors
- Yang, G., Wang, L., Qin, X., Chen, X., Liang, Y., Jin, X., Chen, C., Zhang, W., Pan, W., Li, H.
- Source
- Full text @ Biomed. Opt. Express
|
Principle of the high-throughput 3D imaging system, LS-FIS. (A) Sketch of the LS-FIS optics. L1-L3: lens; CL: cylinder lens; DM: dichroic mirror; M1-M3: mirror; O1-O2: objective; TL1-TL2: tube lens; CAM1-CAM2: camera; C: capillary. See Visualization 1. (B) Sketch of the LS-FIS fluidics. The fluidic flow was driven by a syringe pump between the buffer pool, sample reservoir, and dispense pool and controlled by two three-way valves and two photon-detectors. Val1-Val4: electromagnetic valve; LS: light sheet imaging; BF: bright filed imaging. See Visualization 2. (C) Timeline to reverse the pump, change the flow rate, open/close the valves with the signals from photon-detector PD1 (red) & PD2 (green), and the signal to trigger the imaging camera (blue). DET: detection of larva arrival; Trig: camera trigger. (D) Reconstruction of 3D embryo image by stacking light-sheet images with the correction of larva translational position. |
|
3D image reconstruction with corrected translational distance and image shift. (A) Bright-field images captured by CAM2 to calibrate the embryo movement driven by the flow. Template matching (red box) is applied to determine the translational distance. (B) The calculated translational location of the embryo at each frame in an LS-FIS imaging sequence. (C) 3D reconstruction by resampling along z-axis to a constant distance of 3.99 μm and applying a shift along x-axis between adjacent frames for the 3D light-sheet data. (D) MIP image of an original 3D volume obtained without the correction of translational distance. (E) MIP image from the same set 3D image data with the correction of translational distance. Scale bars represent 50 μm for (D) and (E). |
|
Auto registration of the 3D zebrafish vasculature structure. (A) A typical MIP image of a 3D structure obtained by LS-FIS and the corresponding intensity profile to determine the direction of the head. (B–D) MIP image of the tail region (red box in A) with three different rotation angles. (E) Variances of the serial MIP images with different rotation angles. The peak indicates the rotation angle in lateral view; the minimum indicates the rotation angle in dorsal view. (F, G) Registered MIP images of lateral (F) and dorsal (G) views for the whole zebrafish embryo. Scale bars represent 400 µm for (A, F, G) and 200 µm for (B, C, D), respectively. |
|
Characterization of thickness and resolution of LS-FIS. (A) Light-sheet in LS-FIS captured by the bright-field imaging arm with the long pass filter replaced by an appropriate neutral density filter; (B) Intensity along the blue line at the center of light-sheet in (A) is fitted by Gaussian function showing a FWHM of 9.8 μm. (C) Intensity along the red line at halfway away from the center of light-sheet in (A) is fitted by Gaussian function showing a FWHM of 14.1 μm; (D) Raw image of 100 nm fluorescent beads captured by LS-FIS for calibration of the lateral resolution; (E) Intensity profile along the center of the bead marked in (D). The black curve shows the Gaussian fitting to the profile with FWHM of 2.9 μm. Scale bars represent 200 µm for (A) and 150 µm for (D), respectively. |
|
Whole-larva 3D image of zebrafish vasculature captured with LS-FIS at 5 dpf. (A) 3D rendering of the whole larva. See also Visualization 3. (B) Zoomed-in of the box in (A) showing vessels at the truck. CV: caudal vein; ISV: intersegmental vessels; DLAV: dorsal longitudinal anastomotic vessel; VTA: vertebral artery; DA: dorsal aorta; PCV: posterior cardinal vein. (C) Sectioned view along the dashed line of (B). (D) Zoomed-in of the box in (A) showing the vessels at the head. DLV: dorsal longitudinal vein; MCeV: middle cerebral vein; MsV: mesencephalic vein; HB: hyaloid basket. Scale bars represent sent 100 µm for (B, D) and 50 µm for (C), respectively. |
|
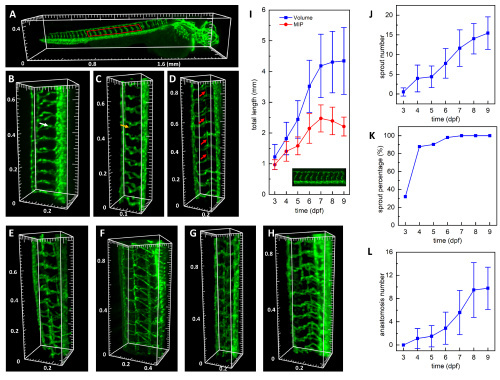
Development process of zebrafish trunk vessels from 3 to 9 dpf. (A) A typical whole larva 3D image at 3 dpf with a red box indicating the trunk vessels at the yolk extension region. See also Visualization 4. (B–H) 3D render of representative trunk vessels from 3 dpf to 9 dpf, respectively. White, yellow, and red arrows indicate the ISV, the sprouting, and anastomosis of the secondary sprout vessel, respectively. (I) The total length of ISVs at the yolk extension region measured from the 3D volume data (blue) and the 2D MIP (red). (J) Number of ISVs with the sprouted secondary vessel. (K) Percentage of larvae with the secondary sprout. (L) The number of anastomosed secondary sprout vessels. Error bars represent the corresponding standard deviation. |
|
Development of zebrafish hyaloid vasculature. (A) Typical head vessel structure; red arrow indicates the hyaloid vessel. See Visualization 5. (B) Schematic of the hyaloid basket from ventral-dorsal view. (C–D) Typical hyaloid vessels at 3 dpf and 7 dpf, respectively. See Visualization 6 and Visualization 7. (E) Projection along the lens axis of a typical hyaloid at 5 dpf showing an ellipse shape (red); blue arrows indicate its long axis and short axis, and their mean value is taken as the diameter of the hyaloid basket. (F) Projection along the V-D axial of the hyaloid showing a basket shape. The blue dashed circle indicates the lens, and brown arrows indicate the depth of the basket from the lower edge to the bottom. (G) Diameters (red) and depths (blue) of hyaloid baskets dependent on time show a continuous growth from 3 to 8 dpf. Scale bar: 40 μm for (E) and (F). D: dorsal, V: ventral, N: nasal, T: temporal, La: lens anterior, Lp: lens posterior. Error bars represent the corresponding standard deviation. |