- Title
-
AI-2/LuxS Quorum Sensing System Promotes Biofilm Formation of Lactobacillus rhamnosus GG and Enhances the Resistance to Enterotoxigenic Escherichia coli in Germ-Free Zebrafish
- Authors
- Deng, Z., Hou, K., Valencak, T.G., Luo, X.M., Liu, J., Wang, H.
- Source
- Full text @ Microbiol Spectr
|
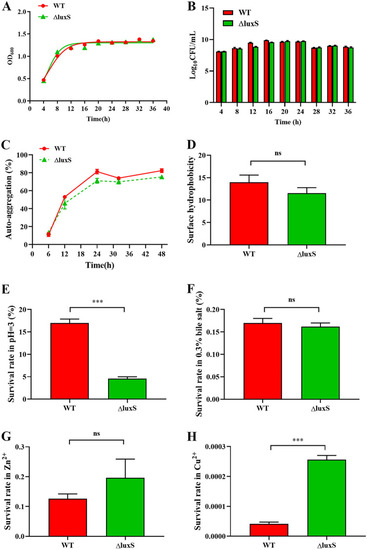
Physiological characteristics and stress resistance in WT and ΔluxS strain. (A–B) WT and ΔluxS were counted after being cultured for 4, 8, 12, 16, 20, 24, 28, 32 and 36 h in MRS. (C) Autoaggregation. (D) Surface hydrophobicity. (E-H) Survival rates in hydrochloric acid, bile salts (0.3%), copper ions and zinc ions (100 mg/L), |
|
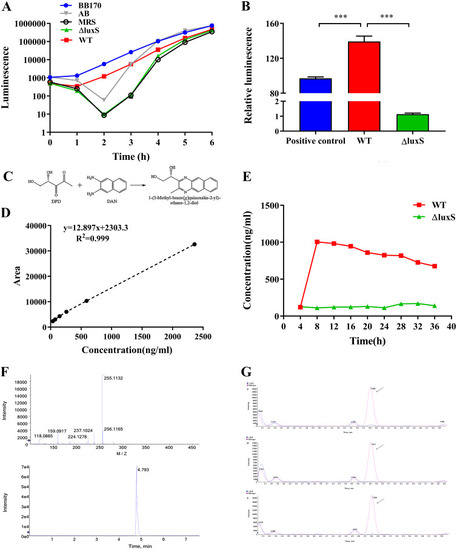
AI-2 activity of LGG wild-type or ΔluxS. (A) Detection of AI-2 activity in the supernatant of the wild-type strain and ΔluxS mutant after inducing |
|
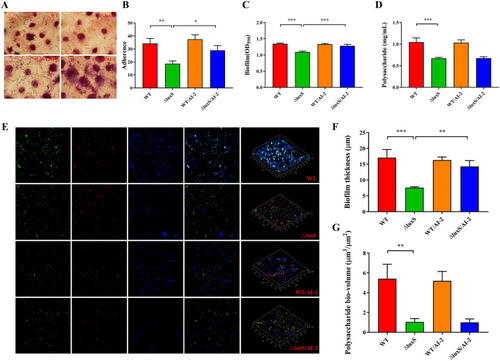
Adherence capacity, biofilm formation and exopolysaccharide (EPS) production of the LGG wild type versus △luxS in the presence or absence of exogenous, synthesized AI-2 (1 μM). (A–B) Adherence of LGG strains to IPEC-J2 cells. Five fields of vision were randomly selected for each slide under oil microscope to calculate the number of bacteria adhered to the surface of the visible cells, |
|
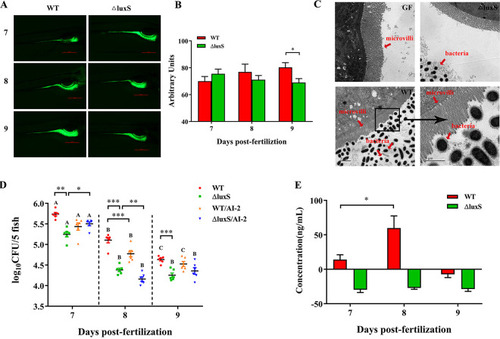
Colonization of germ-free zebrafish larvae by LGG wild-type and ΔluxS strain. (A–B) Analysis of LGG colonization in zebrafish gut on 7, 8 and 9 dpf by fluorescence microscopy, |
|
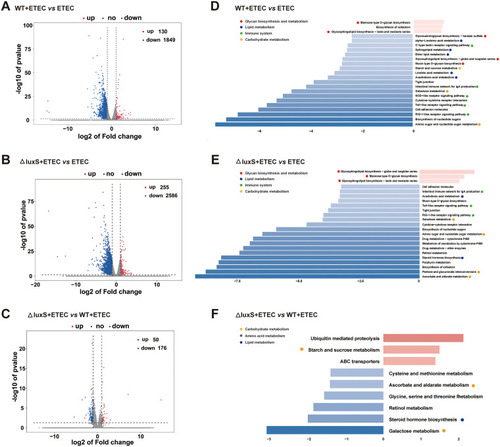
Transcriptomic analysis of zebrafish larvae. (A–C) Volcano plots of DEGs. Log2 (Fold change) ≥1 was set as the threshold. (D–F) Pathway classification based on KEGG enrichment analysis of DEGs. We enriched the differential genes according to Log2 (Fold change) ≥1, upregulated genes |
|
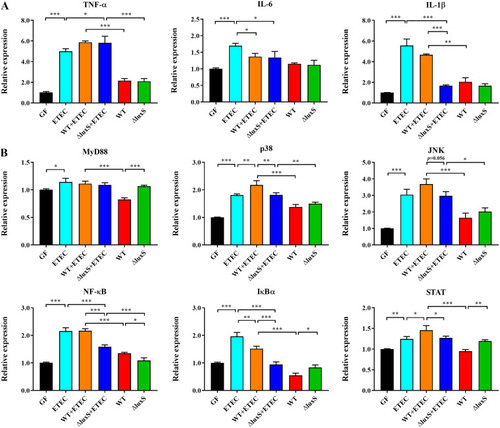
Characterization of inflammatory response in germ-free zebrafish larvae expose to ETEC. (A) mRNA expression of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β). (B) mRNA expression of genes involved in mitogen-activated protein kinases (MAPK), nuclear transcription factor-kappa B (NF-κB) and Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway, |
|
Abstract graphic. Our study compared the difference between the wild-type strain (WT) of |